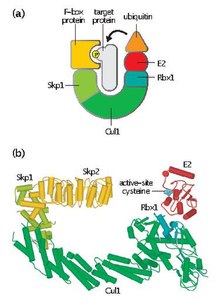
Skp, Cullin, F-box containing complex (or SCF complex) is a multi-protein E3 ubiquitin ligase complex that catalyzes the ubiquitination of proteins destined for 26S proteasomal degradation.[1] Along with the anaphase-promoting complex,[2] SCF has important roles in the ubiquitination of proteins involved in the cell cycle. The SCF complex also marks various other cellular proteins for destruction.[3]
- ^ Ou, Young; Rattner, J.B. (2004), "The Centrosome in Higher Organisms: Structure, Composition, and Duplication", International Review of Cytology, 238, Elsevier: 119–182, doi:10.1016/s0074-7696(04)38003-4, ISBN 978-0-12-364642-2, PMID 15364198
- ^ Fischer, Martin; Dang, Chi V.; DeCaprio, James A. (2018), "Control of Cell Division", Hematology, Elsevier, pp. 176–185, doi:10.1016/b978-0-323-35762-3.00017-2, ISBN 978-0-323-35762-3
- ^ Morgan, David "Protein Degradation in Cell-Cycle Control", The Cell Cycle; Principles of Control 2007