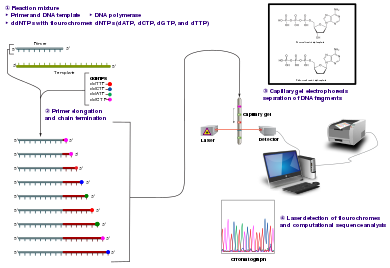
Sanger sequencing is a method of DNA sequencing that involves electrophoresis and is based on the random incorporation of chain-terminating dideoxynucleotides by DNA polymerase during in vitro DNA replication. After first being developed by Frederick Sanger and colleagues in 1977, it became the most widely used sequencing method for approximately 40 years. An automated instrument using slab gel electrophoresis and fluorescent labels was first commercialized by Applied Biosystems in March 1987.[1] Later, automated slab gels were replaced with automated capillary array electrophoresis.[2] More recently, higher volume Sanger sequencing has been replaced by next generation sequencing methods, especially for large-scale, automated genome analyses. However, the Sanger method remains in wide use for smaller-scale projects and for validation of deep sequencing results. It still has the advantage over short-read sequencing technologies (like Illumina) in that it can produce DNA sequence reads of > 500 nucleotides and maintains a very low error rate with accuracies around 99.99%.[3] Sanger sequencing is still actively being used in efforts for public health initiatives such as sequencing the spike protein from SARS-CoV-2[4] as well as for the surveillance of norovirus outbreaks through the Center for Disease Control and Prevention's (CDC) CaliciNet surveillance network.[5]
- ^ Chait, Edward; Page, Guy; Hunkapiller, Michael (1988). "Battle of the DNA sequencers". Nature. 333 (6172): 477–478. doi:10.1038/333477a0. ISSN 0028-0836.
- ^ Zhang, J (1999-12-15). "A multiple-capillary electrophoresis system for small-scale DNA sequencing and analysis". Nucleic Acids Research. 27 (24): 36e–36. doi:10.1093/nar/27.24.e36. PMC 148759. PMID 10572188.
- ^ Shendure J, Ji H (October 2008). "Next-generation DNA sequencing". Nature Biotechnology. 26 (10): 1135–1145. doi:10.1038/nbt1486. PMID 18846087. S2CID 6384349.
- ^ Daniels RS, Harvey R, Ermetal B, Xiang Z, Galiano M, Adams L, McCauley JW (November 2021). "A Sanger sequencing protocol for SARS-CoV-2 S-gene". Influenza and Other Respiratory Viruses. 15 (6): 707–710. doi:10.1111/irv.12892. PMC 8447197. PMID 34346163.
- ^ Vega E, Barclay L, Gregoricus N, Williams K, Lee D, Vinjé J (August 2011). "Novel surveillance network for norovirus gastroenteritis outbreaks, United States". Emerging Infectious Diseases. 17 (8): 1389–1395. doi:10.3201/eid1708.101837. PMC 3381557. PMID 21801614.