| Classification | Colorimetric method |
|---|---|
| Analytes | Aldehydes |
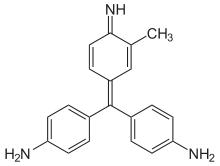
The Schiff test is an early organic chemistry named reaction developed by Hugo Schiff,[1] and is a relatively general chemical test for detection of many organic aldehydes that has also found use in the staining of biological tissues.[2] The Schiff reagent is the reaction product of a dye formulation such as fuchsin and sodium bisulfite; pararosaniline (which lacks an aromatic methyl group) and new fuchsin (which is uniformly mono-methylated ortho to the dye's amine functionalities) are not dye alternatives with comparable detection chemistry.
In its use as a qualitative test for aldehydes, the unknown sample is added to the decolorized Schiff reagent; when aldehyde is present a characteristic magenta color develops. Schiff-type reagents are used for various biological tissue staining methods, e.g. Feulgen stain and periodic acid-Schiff stain. Human skin also contains aldehyde functional groups in the termini of saccharides and so is stained as well.
- ^ See:
- Schiff, Hugo (1866) "Eine neue Reihe organischer Diaminen. Zeite Abteilung." (A new series of organic diamines. Second part.), Justus Liebigs Annalen der Chemie, 140 : 92–137. [in German] From p. 132: "Das Rosanilinsulfit verbindet sich nicht direct mit den Aldehyden. Schüttelt man die rothe Lösung des krystallisirten neutralen Sulfits oder auch die nach obiger Weise bereitete leukanilinhaltige gelbe Lösung mit irgend einem flüssigen Aldehyd, so erhält man sogleich eine rothe Lösung, und diese Farbe verwandelt sich allmählich in ein je nach dem angewandten Aldehyd helleres oder dunkleres Violettblau." (Rosaniline [i.e., fuchsine] sulfite does not combine directly with aldehydes. If one shakes, with any liquid aldehyde, a red solution of the crystallized neutral sulfite [of fuchsine] or even the yellow solution which was prepared in the above-mentioned way and which contains leukaniline [i.e., fuchsine treated with aqueous sulfurous acid], then one obtains at once a red solution, and this color is transformed into a lighter or darker violet blue, depending on the aldehyde that's used.)
- See also: Schiff, Ugo (1866) "Sopra una nova serie di basi organiche" (On a new series of organic bases), Giornale di scienze naturali ed economiche, 2 : 201–257. [in Italian] From p. 253: "Il solfito di rosanilina non si combina direttamente colle aldeidi. Quando si agita una soluzione solforosa di idrato o di acetato di rosanilina con un'aldeide qualunque, allora la soluzione gialla ássume subito un colore rosso che poco a poco si trasforma in un bel violetto più or meno intenso a secondo l'aldeide applicata." (Rosaniline sulphite does not combine directly with aldehydes. When a sulfurous solution of rosaniline hydrate or rosaniline acetate is shaken with any aldehyde, then the yellow solution immediately becomes a red color that little by little transforms into a fine violet, [which is] more or less intense according to the aldehyde [that's] used.)
- See also: Schiff, Hugo (1867) "Dérivés de la rosaniline" (Derivatives of rosanaline [i.e., fuchsine]), Comptes rendus, 64 : 182–184. [in French] From p. 182: " … si l'on agite une solution sulfureuse diluée, soit de sulfite, soit de tout autre sel de rosaniline, avec quelques gouttes d'un aldéhyde, alors il se dégage de l'acide sulfureux, la solution se colore d'abord en rouge, puis en violet, et peu à peu il se forme un précipité constitué de petites écailles cristallines d'un violet cuivré." ( … if one shakes a dilute sulfurous solution either of the sulphite or any other salt of rosaniline [i.e., fuchsine] with a few drops of an aldehyde, then sulphurous acid is evolved; the solution is first colored red, then violet, and gradually a precipitate is formed consisting of small crystalline flakes of a coppery violet.)
- ^ Histology, cell and tissue biology 5th Ed., 1983 ISBN 0-333-35406-0