| |||
| Names | |||
|---|---|---|---|
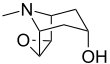
| IUPAC name
6β,7β-Epoxytropan-3α-ol
| |||
| Systematic IUPAC name
(1R,2R,4S,5S,7s)-9-Methyl-3-oxa-9-azatricyclo[3.3.1.02,4]nonan-7-ol | |||
| Other names
6,7-Epoxytropine; Scopanol; Scopin; 6β,7β-Epoxy-1αH,5αH-tropan-3α-ol
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEMBL | |||
| ChemSpider | |||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C8H13NO2 | |||
| Molar mass | 155.197 g·mol−1 | ||
| Melting point | 75 to 76 °C (167 to 169 °F; 348 to 349 K)[1] | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Scopine is a tropane alkaloid found in a variety of plants including Mandragora root,[2] Senecio mikanioides (Delairea odorata),[3] Scopolia carniolica,[4] and Scopolia lurida.[5]
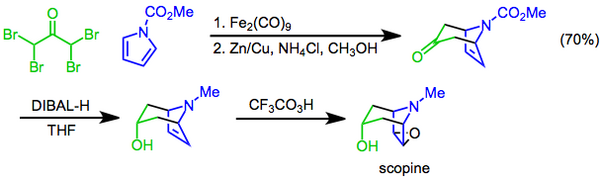
Scopine can be prepared by the hydrolysis of scopolamine.[1][6][7] It can also be prepared in three steps from N-methoxycarbonylpyrrole and 1,1,3,3-tetrabromoacetone; the reagents are combined in a [4+3] cycloaddition, followed by a diastereoselective reduction with diisobutylaluminum hydride, and finally a Prilezhaev epoxidation with trifluoroperacetic acid affords scopine.[8]
- ^ a b Werner, Gottfried; Schmidt, K.-H. (1967). "Die darstellung von scopin aus scopolamin". Tetrahedron Letters. 8 (14): 1283–1284. doi:10.1016/S0040-4039(00)90685-3. PMID 6044210.
- ^ Staub, H. (1962). "The chemical constituents of the Mandragora root. II. The alkaloids". Helvetica Chimica Acta. 45 (7): 2297–2305. doi:10.1002/hlca.19620450703.
- ^ Adams, Roger; Gianturco, Maurizio (1957). "Senecio alkaloids: mikanoidine, the alkaloid from Senecio mikanoides". Journal of the American Chemical Society. 79: 166–169. doi:10.1021/ja01558a045.
- ^ Bendik, I.; Bauerova, O.; Bauer, S.; Mokry, J.; Tomko, J. (1958). "Alkaloids from Scopolia carniolica". Chemicke Zvesti. 12: 181–184.
- ^ Szymanska, Miroslawa (1967). "Alkaloids in Scopolia lurida. Chromatographic analysis. Isolation of cuscohygrine". Acta Poloniae Pharmaceutica. 24 (1): 59–64.
- ^ Meinwald, J.; Chapman, O. L. (1957). "Alkaline hydrolysis of scopolamine methoxymethochloride: a new route to scopine". Journal of the American Chemical Society. 79 (3): 665–666. doi:10.1021/ja01560a042.
- ^ Willstatter, Richard; Berner, Endre (1923). "Hydrolysis of scopolamine". Berichte der Deutschen Chemischen Gesellschaft B. 56 (5): 1079–1082. doi:10.1002/cber.19230560515.
- ^ Hayakawa, Y.; Baba, Y.; Makino, S.; Noyori, R. (1978). "Carbon-carbon bond formation promoted by transition metal carbonyls. 19. General synthesis of tropane alkaloids via the polybromo ketone-iron carbonyl reaction". J. Am. Chem. Soc. 100 (6): 1786–1791. doi:10.1021/ja00474a021.