| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Selenium hexafluoride
| |||
| Other names
Selenium(VI) fluoride, Selenium fluoride
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
| ECHA InfoCard | 100.149.506 | ||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
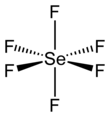
| SeF6 | |||
| Molar mass | 192.9534 g/mol | ||
| Appearance | colourless gas | ||
| Density | 0.007887 g/cm3[1] | ||
| Melting point | −39 °C (−38 °F; 234 K) | ||
| Boiling point | −34.5 °C (−30.1 °F; 238.7 K) sublimes | ||
| insoluble | |||
| Vapor pressure | >1 atm (20°C)[2] | ||
| −51.0·10−6 cm3/mol | |||
Refractive index (nD)
|
1.895 | ||
| Structure | |||
| Orthorhombic, oP28 | |||
| Pnma, No. 62 | |||
| octahedral (Oh) | |||
| 0 | |||
| Thermochemistry | |||
Std enthalpy of
formation (ΔfH⦵298) |
-1030 kJ/mol[3] | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards
|
toxic, corrosive | ||
| NFPA 704 (fire diamond) | |||
| Lethal dose or concentration (LD, LC): | |||
LCLo (lowest published)
|
10 ppm (rat, 1 hr) 10 ppm (mouse, 1 hr) 10 ppm (guinea pig, 1 hr)[4] | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible)
|
TWA 0.05 ppm (0.4 mg/m3)[2] | ||
REL (Recommended)
|
TWA 0.05 ppm[2] | ||
IDLH (Immediate danger)
|
2 ppm[2] | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Selenium hexafluoride is the inorganic compound with the formula SeF6. It is a very toxic colourless gas described as having a "repulsive" odor.[5] It is not widely encountered and has no commercial applications.[6]
- ^ Lide, D. R., ed. (2005). CRC Handbook of Chemistry and Physics (86th ed.). Boca Raton (FL): CRC Press. ISBN 0-8493-0486-5.
- ^ a b c d NIOSH Pocket Guide to Chemical Hazards. "#0551". National Institute for Occupational Safety and Health (NIOSH).
- ^ Wiberg, E.; Holleman, A. F. (2001). Inorganic Chemistry. Elsevier. ISBN 0-12-352651-5.
- ^ "Selenium hexafluoride". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- ^ "Material Safety" (PDF). Retrieved 2010-07-24.
- ^ Langner, B. E. "Selenium and Selenium Compounds". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a23_525. ISBN 978-3527306732.