| |

| |
| Names | |
|---|---|
| IUPAC name
Selenium tetrachloride
| |
| Other names
Selenium(IV) chloride, tetrachloro-λ4-selane
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.030.036 |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| SeCl4 | |
| Molar mass | 220.771 g/mol |
| Appearance | white to yellow crystals |
| Density | 2.6 g/cm3, solid |
| Melting point | sublimes at 191.4 °C[1] |
| decomposes in water | |
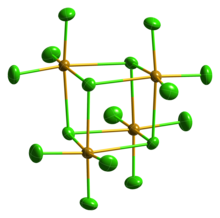
| Structure | |
| Monoclinic, mS80 | |
| C12/c1, No. 15 | |
| Seesaw (gas phase)[citation needed] | |
| Hazards[2] | |
| GHS labelling: | |
  
| |
| Danger | |
| H301, H331, H373, H410 | |
| P260, P261, P264, P270, P271, P273, P301+P310, P304+P340, P311, P314, P321, P330, P391, P403+P233, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Flash point | non-flammable |
| Related compounds | |
Other anions
|
Selenium tetrafluoride Selenium tetrabromide Selenium dioxide |
Other cations
|
Dichlorine monoxide Sulfur tetrachloride Tellurium tetrachloride |
Related compounds
|
Selenium dichloride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Selenium tetrachloride is the inorganic compound composed with the formula SeCl4. This compound exists as yellow to white volatile solid. It is one of two commonly available selenium chlorides, the other example being selenium monochloride, Se2Cl2. SeCl4 is used in the synthesis of other selenium compounds.
- ^ Lide, David R. (1998). Handbook of Chemistry and Physics (87 ed.). Boca Raton, Florida: CRC Press. p. 487. ISBN 0-8493-0594-2. Retrieved 2008-07-02.
- ^ "323527 Selenium tetrachloride". Sigma-Aldrich. Retrieved 2008-07-02.
