 | |
| Clinical data | |
|---|---|
| Other names | LY-450139 |
| Routes of administration | By mouth |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Metabolism | CYP3A4, 3A5[1] |
| Elimination half-life | 2.4 hours in circulation |
| Excretion | 87% renal (44% unchanged, 43% as metabolites) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.318.475 |
| Chemical and physical data | |
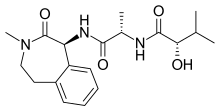
| Formula | C19H27N3O4 |
| Molar mass | 361.442 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Semagacestat (LY-450139) was a candidate drug for a causal therapy against Alzheimer's disease. It was originally developed by Eli Lilly and Elan, and clinical trials were conducted by Eli Lilly. Phase III trials included over 3000 patients,[2][3] but in August 2010, a disappointing interim analysis, in which semagacestat performed worse than the placebo, led to the trials being stopped.