| |||
|
| |||

| |||
| Names | |||
|---|---|---|---|
| Other names
silicon tetraiodide
Tetraiodosilane | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
| ECHA InfoCard | 100.033.355 | ||
| EC Number |
| ||
PubChem CID
|
|||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| SiI4 | |||
| Molar mass | 535.7034 g/mol | ||
| Appearance | white powder | ||
| Density | 4.198 g/cm3 | ||
| Melting point | 120.5 °C (248.9 °F; 393.6 K) | ||
| Boiling point | 287.4 °C (549.3 °F; 560.5 K) | ||
| reacts | |||
| Solubility in organic solvents | soluble | ||
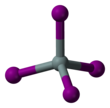
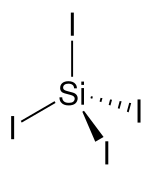
| Structure | |||
| tetrahedral | |||
| Hazards | |||
| GHS labelling: | |||
   
| |||
| Danger | |||
| H301, H311, H314, H317, H334, H360 | |||
| P201, P202, P260, P261, P264, P270, P272, P280, P281, P285, P301+P310, P301+P330+P331, P302+P352, P303+P361+P353, P304+P340, P304+P341, P305+P351+P338, P308+P313, P310, P312, P321, P322, P330, P333+P313, P342+P311, P361, P363, P405, P501 | |||
| NFPA 704 (fire diamond) | |||
| Related compounds | |||
Other anions
|
Silicon tetrafluoride Silicon tetrachloride Silicon tetrabromide | ||
Other cations
|
Carbon tetraiodide Germanium tetraiodide Tin(IV) iodide | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Silicon tetraiodide is the chemical compound with the formula SiI4. It is a tetrahedral molecule with Si-I bond lengths of 2.432(5) Å.[1]
SiI4 is a precursor to silicon amides of the formula Si(NR2)4 (R = alkyl).[2] It has also been of interest in the manufacture and etching of silicon in microelectronics.
- ^ Kolonits, Maria; Hargittai, Magdolna (1998). "Molecular Structure of Silicon Tetraiodide". Structural Chemistry. 9 (5): 349–352. doi:10.1023/A:1022462926682. S2CID 96658381.
- ^ Banerjee, Chiranjib; Wade, Casey R.; Soulet, Axel; Jursich, Gregory; McAndrew, James; Belot, John A. (2006). "Direct syntheses and complete characterization of halide-free tetrakis(dialkylamino)silanes". Inorganic Chemistry Communications. 9 (7): 761. doi:10.1016/j.inoche.2006.04.027.