 | |
| Clinical data | |
|---|---|
| Other names | PTI-125, PTI-910 |
| ATC code |
|
| Pharmacokinetic data | |
| Elimination half-life | 4.5 hrs[1] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
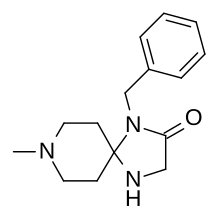
| Formula | C15H21N3O |
| Molar mass | 259.353 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Simufilam (PTI-125) is an experimental medication for the treatment of Alzheimer's disease.[2][3] It is being developed by the American pharmaceutical firm Cassava Sciences. The drug is in phase III clinical trials as of October 2023. There are two phase III clinical studies: RETHINK-ALZ, a 52-week trial, is set to complete in 2024,[4] and REFOCUS-ALZ, spanning 76 weeks, is projected to finish in 2025.[5]
The US Food and Drug Administration (FDA) received a citizen petition in August 2021 to stop the clinical trials and investigate simufilam. Other scientists have questioned the preclinical results, citing the small sample size, alleged methodological flaws in an in vitro technique, alleged manipulations of western blot images and potential conflict of interest.[6][7][8]
After the FDA said that the citizen petition was the improper procedure to request an investigation, Reuters reported in July 2022 that a criminal investigation of Cassava Sciences was started by the United States Department of Justice (DOJ) over research results related to the experimental drug.[9] The U.S. Securities and Exchange Commission (SEC), the U.S. National Institutes of Health (NIH), and City University of New York (CUNY) were also investigating whether Cassava or individuals manipulated data.[10] In June 2024, Wang was charged by the United States Department of Justice (DOJ) with fraud for falsifying data on $16 million in NIH grant applications related to simufilam.[11][12][13][14]
- ^ Cite error: The named reference
Wang22was invoked but never defined (see the help page). - ^ "A Phase 3, Randomized, Double-blind, Placebo-controlled, Parallel-group, 52-week Study Evaluating the Safety and Efficacy of Simufilam 100 mg Tablets in Subjects with Mild-to-Moderate Alzheimer's Disease". May 25, 2022. Archived from the original on June 6, 2022. Retrieved June 6, 2022.
- ^ "A Phase 3, Randomized, Double-blind, Placebo-controlled, Parallel-group, 76-week Study Evaluating the Safety and Efficacy of Two Doses of Simufilam in Subjects with Mild-to-Moderate Alzheimer's Disease". June 2, 2022.
- ^ "A Phase 3, Randomized, Double-blind, Placebo-controlled, Parallel-group, 52-week Study Evaluating the Safety and Efficacy of Simufilam 100 mg Tablets in Subjects With Mild-to-Moderate Alzheimer's Disease | Alzheimers.gov". www.nia.nih.gov.
- ^ "A Phase 3, Randomized, Double-blind, Placebo-controlled, Parallel-group, 76-week Study Evaluating the Safety and Efficacy of Two Doses of Simufilam in Subjects With Mild-to-Moderate Alzheimer's Disease | Alzheimers.gov". www.nia.nih.gov.
- ^ Mandavilli A (April 18, 2022). "Scientists Question Data Behind an Experimental Alzheimer's Drug". The New York Times. Archived from the original on April 27, 2022. Retrieved April 28, 2022.
- ^ Piller C (October 12, 2023). Co-developer of Cassava's potential Alzheimer's drug cited for 'egregious misconduct' (Report). Science. doi:10.1126/science.adl3444.
- ^ Mandavilli A (October 14, 2023). "Scientists Investigating Alzheimer's Drug Faulted in Leaked Report". The New York Times. Archived from the original on October 15, 2023. Retrieved October 15, 2023.
- ^ Taylor M, Spector M (July 27, 2022). "Exclusive: Cassava Sciences faces U.S. criminal probe tied to Alzheimer's drug, sources say". Reuters. Archived from the original on July 30, 2022. Retrieved July 31, 2022.
- ^ Cite error: The named reference
Michaelswas invoked but never defined (see the help page). - ^ "Cassava Sciences Issues Statement on Former Science Advisor" (Press release). Cassava Sciences. June 28, 2024. Retrieved June 29, 2024.
- ^ Mandavilli A (June 28, 2024). "Embattled Alzheimer's Researcher Is Charged With Fraud". The New York Times. Retrieved June 29, 2024.
- ^ Wosen J (June 28, 2024). "Cassava Sciences collaborator charged with defrauding NIH in grants supporting its Alzheimer's drug". Stat News. Retrieved June 29, 2024.
- ^ Walker J (June 28, 2024). "Cassava Sciences Adviser Indicted on Fraud Charges". The Wall Street Journal. Retrieved June 29, 2024.