| |
| Names | |
|---|---|
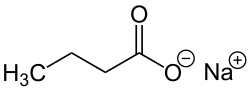
| Preferred IUPAC name
Sodium butanoate | |
| Other names
Sodium butyrate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.005.326 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C4H7NaO2 | |
| Molar mass | 110.088 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Sodium butyrate is a compound with formula Na(C3H7COO). It is the sodium salt of butyric acid. It has various effects on cultured mammalian cells including inhibition of proliferation, induction of differentiation and induction or repression of gene expression.[1] As such, it can be used in lab to bring about any of these effects. Specifically, butyrate treatment of cells results in histone hyperacetylation, and butyrate itself inhibits class I histone deacetylase (HDAC) activity,[2] specifically HDAC1, HDAC2, HDAC3, and butyrate can be used in determining histone deacetylene in chromatin structure and function. Inhibition of HDAC activity is estimated to affect the expression of only 2% of mammalian genes.[3]
In the lab, sodium butyrate is usually found as a white, water-soluble, crystalline solid. The chemical is notable for having a very strong, unpleasant smell that lingers.[4] When working with sodium butyrate, gloves, eye protection and respiratory masks are advised for safety purposes.[5]
The compound is found in human diet, notably produced in large amounts from dietary fiber in the gut and present in Parmesan cheese and butter.[6] Nevertheless, the most common source of sodium butyrate in the gut is from consumption of legumes.[7]
- ^ Kruh, Jacques (1981). "Effects of sodium butyrate, a new pharmacological agent, on cells in culture". Molecular and Cellular Biochemistry. 42 (2): 65–82. doi:10.1007/BF00222695. PMID 6174854. S2CID 24214720.
- ^ Candido, E; Reeves, Raymond; Davie, James R. (1978). "Sodium butyrate inhibits histone deacetylation in cultured cells". Cell. 14 (1): 105–13. doi:10.1016/0092-8674(78)90305-7. PMID 667927. S2CID 33206068.
- ^ Davie, James R. (2003). "Inhibition of Histone Deacetylase Activity by Butyrate". The Journal of Nutrition. 133 (7 Suppl): 2485S–2493S. doi:10.1093/jn/133.7.2485s. PMID 12840228.
- ^ "Sodium butanoate | 156-54-7". Chemicalbook.com. Retrieved 2016-05-31.
- ^ "Sodium butyrate ≥98.5% (GC) | Sigma-Aldrich". Sigmaaldrich.com. Retrieved 2016-05-31.
- ^ Li, Huating; Gao, Zhanguo; Zhang, Jin; Ye, Xin; Xu, Aimin; Ye, Jianping; Jia, Weiping (1 April 2012). "Sodium Butyrate Stimulates Expression of Fibroblast Growth Factor 21 in Liver by Inhibition of Histone Deacetylase 3". Diabetes. 61 (4): 797–806. doi:10.2337/db11-0846. PMC 3314370. PMID 22338096. Retrieved 27 November 2021 – via diabetes.diabetesjournals.org.
- ^ Buettner, Dan (22 May 2015). "Want Great Longevity and Health? It Takes a Village". Wsj.com. Retrieved 27 November 2021.