 Sodium diacetate
| |
| Names | |
|---|---|
| IUPAC name
Sodium diacetate
| |
| Other names
Sodium diacetate (anhydrous); Sodium hydrogen acetate; Sodium acid acetate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.004.378 |
| MeSH | diacetate sodium diacetate |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C4H7NaO4 | |
| Molar mass | 142.086 g·mol−1 |
| Appearance | White powder |
| Odor | Acetic acid (vinegar) odor |
| 1 g/mL | |
| Solubility in alcohol | Slightly |
| Solubility in ether | Insoluble |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Inhalation hazards
|
Irritant[1] |
Eye hazards
|
Irritant[1] |
| GHS labelling:[1] | |
 
| |
| Danger | |
| H318, H319 | |
| P264, P280, P305+P351+P338, P310, P337+P313 | |
| Flash point | >150 °C (302 °F)[1] |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
>2,000 mg/kg (rat, dermal), 5,600 mg/kg (rat, oral) |
| Safety data sheet (SDS) | PubChem sodium diacetate LCSS |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
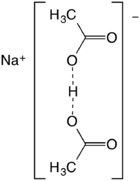
Sodium diacetate is a compound with formula NaH(C
2H
3O
2)
2. It is a salt of acetic acid. It is a colorless solid that is used in seasonings and as an antimicrobial agent.
- ^ a b c d PubChem. "Sodium diacetate". PubChem. Retrieved 2019-10-24.