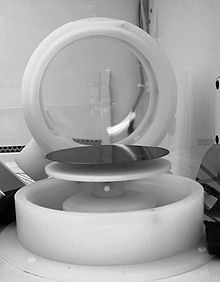
Spin coating is a procedure used to deposit uniform thin films onto flat substrates. Usually a small amount of coating material in liquid form is applied on the center of the substrate, which is either spinning at low speed or not spinning at all. The substrate is then rotated at speeds up to 10,000 rpm to spread the coating material by centrifugal force. A machine used for spin coating is called a spin coater, or simply spinner.[1]
Rotation is continued while the fluid spins off the edges of the substrate, until the desired thickness of the film is achieved. The applied solvent is usually volatile, and simultaneously evaporates. The higher the angular speed of spinning, the thinner the film. The thickness of the film also depends on the viscosity and concentration of the solution, and the solvent.[2] Pioneering theoretical analysis of spin coating was undertaken by Emslie et al.,[3] and has been extended by many subsequent authors (including Wilson et al.,[4] who studied the rate of spreading in spin coating; and Danglad-Flores et al.,[5] who found a universal description to predict the deposited film thickness).
Spin coating is widely used in microfabrication of functional oxide layers on glass or single crystal substrates using sol-gel precursors, where it can be used to create uniform thin films with nanoscale thicknesses.[6] It is used intensively in photolithography, to deposit layers of photoresist about 1 micrometre thick. Photoresist is typically spun at 20 to 80 revolutions per second for 30 to 60 seconds. It is also widely used for the fabrication of planar photonic structures made of polymers.
One advantage to spin coating thin films is the uniformity of the film thickness. Owing to self-leveling, thicknesses do not vary more than 1%. The thickness of films produced in this manner may also affect the optical properties of such materials. This is important for electrochemical testing, specifically when recording absorbance readings from Ultraviolet-visible Spectroscopy, since thicker films have lower optical transmittance and typically do not allow light to shine through in comparison to thinner films allowing light to go through before the optical density of the film becomes too low. Additionally, films with lower absorbance quality are not as ideal of candidates for processes such as Cyclic Voltammetry because the low absorbance hinders electrochemical tuning of cations when in an electrochemical cell. Thinner films in this regard have more desirable optical properties that can be tuned for energy storage technologies because of their spin coated influenced properties.[7] However, spin coating thicker films of polymers and photoresists can result in relatively large edge beads whose planarization has physical limits.[8]
- ^ Cohen, Edward; Lightfoot, E. J. (2011). "Coating Processes". Kirk-Othmer Encyclopedia of Chemical Technology. New York: John Wiley. doi:10.1002/0471238961.1921182203150805.a01.pub3. ISBN 978-0471238966.
- ^ Scriven, L. E. (1988). "Physics and Applications of DIP Coating and Spin Coating". MRS Proceedings. 121. Cambridge University Press (CUP): 717. doi:10.1557/proc-121-717. ISSN 1946-4274.
- ^ Emslie, A. G.; Bonner, F. T.; Peck, L. G. (1958). "Flow of a viscous liquid on a rotating disk". J. Appl. Phys. 29 (5): 858–862. Bibcode:1958JAP....29..858E. doi:10.1063/1.1723300.
- ^ Wilson, S. K.; Hunt, R.; Duffy, B. R. (2000). "The rate of spreading in spin coating". J. Fluid Mech. 413 (1): 65–88. Bibcode:2000JFM...413...65W. doi:10.1017/S0022112000008089. S2CID 14585243.
- ^ Danglad-Flores, J.; Eickelmann, S.; Riegler, H. (2018). "Deposition of polymer films by spin casting: A quantitative analysis". Chem. Eng. Sci. 179: 257–264. doi:10.1016/j.ces.2018.01.012. hdl:21.11116/0000-0000-2D6C-6.
- ^ Hanaor, D.A.H.; Triani, G.; Sorrell, C.C. (2011). "Morphology and photocatalytic activity of highly oriented mixed phase titanium dioxide thin films". Surface and Coatings Technology. 205 (12). Elsevier BV: 3658–3664. arXiv:1303.2741. doi:10.1016/j.surfcoat.2011.01.007. ISSN 0257-8972. S2CID 96130259.
- ^ "What Is Spin Coating?". Inseto. November 4, 2020. Retrieved 2023-05-24.
- ^ Arscott, Steve (2020). "The limits of edge bead planarization and surface levelling in spin-coated liquid films". Journal of Micromechanics and Microengineering. 30 (2): 025003. doi:10.1088/1361-6439/ab60be. hdl:20.500.12210/44092. S2CID 214580612.