| |

| |
| Names | |
|---|---|
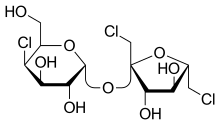
| IUPAC name
1,6-Dichloro-1,6-dideoxy-β-D-fructofuranosyl 4-chloro-4-deoxy-α-D-galactopyranoside
| |
| Systematic IUPAC name
(2R,3R,4R,5R,6R)-2-{[(2R,3S,4S,5S)-2,5-Bis(chloromethyl)-3,4-dihydroxyoxolan-2-yl]oxy}-5-chloro-6-(hydroxymethyl)oxane-3,4-diol | |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.054.484 |
| EC Number |
|
| E number | E955 (glazing agents, ...) |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C12H19Cl3O8 | |
| Molar mass | 397.63 g·mol−1 |
| Appearance | Off-white to white powder |
| Odor | Odorless |
| Density | 1.69 g/cm3 |
| Melting point | 125 °C (257 °F; 398 K) |
| 283 g/L (20 °C) | |
| Acidity (pKa) | 12.52±0.70 |
| Hazards | |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Sucralose is an artificial sweetener and sugar substitute. As the majority of ingested sucralose is not metabolized by the body, it adds very little food energy (14 kJ [3.3 kcal] per gram).[3] In the European Union, it is also known under the E number E955. It is produced by chlorination of sucrose, selectively replacing three of the hydroxy groups—in the C1 and C6 positions of the fructose portion and the C4 position of the glucose portion—to give a 1,6-dichloro-1,6-dideoxyfructose–4-chloro-4-deoxygalactose disaccharide. Sucralose is about 600 times sweeter than sucrose (table sugar),[4][5] 3 times as sweet as both aspartame and acesulfame potassium, and 2 times as sweet as sodium saccharin.[4]
The commercial success of sucralose-based products stems from its favorable comparison to other low-calorie sweeteners in terms of taste, stability, and safety.[4][6] It is commonly sold under the Splenda brand name.[4]
- ^ Merck Index, 11th Edition, 8854.
- ^ Anonymous. Scifinder – Substance Detail for 56038-13-2, 30 October 2010.
- ^ "Sucralose nutrition information for a one gram portion (pick list)". FoodData Central, US Department of Agriculture. 1 April 2019. Retrieved 11 May 2024.
- ^ a b c d "Aspartame and Other Sweeteners in Food". US Food and Drug Administration. 25 September 2024. Retrieved 17 November 2024.
- ^ Friedman MA (3 April 1998). "Food Additives Permitted for Direct Addition to Food for Human Consumption; Sucralose" (PDF). Federal Register: 21 CFR Part 172, Docket No. 87F-0086.
Lead Deputy Commissioner for the FDA
- ^ "A Report on Sucralose from the Food Sanitation Council". The Japan Food Chemical Research Foundation. Archived from the original on 15 October 2012.
