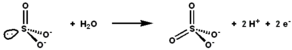
Sulfite oxidase (EC 1.8.3.1 ) is an enzyme in the mitochondria of all eukaryotes , with exception of the yeasts.[citation needed It oxidizes sulfite to sulfate and, via cytochrome c , transfers the electrons produced to the electron transport chain , allowing generation of ATP in oxidative phosphorylation .[ 5] [ 6] [ 7] sulfur -containing compounds and the sulfate is excreted.
Sulfite oxidase is a metallo-enzyme that utilizes a molybdopterin cofactor and a heme group (in the case of animals). It is one of the cytochromes b 5 and belongs to the enzyme super-family of molybdenum oxotransferases that also includes DMSO reductase , xanthine oxidase , and nitrite reductase .
In mammals, the expression levels of sulfite oxidase is high in the liver, kidney, and heart, and very low in spleen, brain, skeletal muscle, and blood.
^ a b c GRCh38: Ensembl release 89: ENSG00000139531 – Ensembl , May 2017^ a b c GRCm38: Ensembl release 89: ENSMUSG00000049858 – Ensembl , May 2017^ "Human PubMed Reference:" . National Center for Biotechnology Information, U.S. National Library of Medicine .^ "Mouse PubMed Reference:" . National Center for Biotechnology Information, U.S. National Library of Medicine .^ D'Errico G, Di Salle A, La Cara F, Rossi M, Cannio R (January 2006). "Identification and characterization of a novel bacterial sulfite oxidase with no heme binding domain from Deinococcus radiodurans" . J. Bacteriol . 188 (2): 694–701. doi :10.1128/JB.188.2.694-701.2006 . PMC 1347283 PMID 16385059 . ^ Tan WH, Eichler FS, Hoda S, Lee MS, Baris H, Hanley CA, Grant PE, Krishnamoorthy KS, Shih VE (September 2005). "Isolated sulfite oxidase deficiency: a case report with a novel mutation and review of the literature". Pediatrics . 116 (3): 757–66. doi :10.1542/peds.2004-1897 . PMID 16140720 . S2CID 6506338 . ^ Cohen HJ, Betcher-Lange S, Kessler DL, Rajagopalan KV (December 1972). "Hepatic sulfite oxidase. Congruency in mitochondria of prosthetic groups and activity" . J. Biol. Chem . 247 (23): 7759–66. doi :10.1016/S0021-9258(19)44588-2 PMID 4344230 .