| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Sulfur(IV) fluoride
| |||
| Other names
Sulfur tetrafluoride
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.029.103 | ||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 2418 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| SF4 | |||
| Molar mass | 108.07 g/mol | ||
| Appearance | colorless gas | ||
| Density | 1.95 g/cm3, −78 °C | ||
| Melting point | −121.0 °C | ||
| Boiling point | −38 °C | ||
| reacts | |||
| Vapor pressure | 10.5 atm (22 °C)[1] | ||
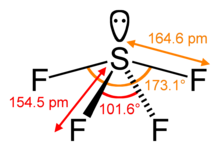
| Structure | |||
| Seesaw (C2v) | |||
| 0.632 D[2] | |||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards
|
highly reactive and toxic gas | ||
| NFPA 704 (fire diamond) | |||
| NIOSH (US health exposure limits): | |||
PEL (Permissible)
|
none[1] | ||
REL (Recommended)
|
C 0.1 ppm (0.4 mg/m3)[1] | ||
IDLH (Immediate danger)
|
N.D.[1] | ||
| Safety data sheet (SDS) | ICSC 1456 | ||
| Related compounds | |||
Other anions
|
Sulfur dichloride Disulfur dibromide Sulfur trifluoride | ||
Other cations
|
Oxygen difluoride Selenium tetrafluoride Tellurium tetrafluoride | ||
Related sulfur fluorides
|
Disulfur difluoride Sulfur difluoride Disulfur decafluoride Sulfur hexafluoride | ||
Related compounds
|
Thionyl fluoride | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Sulfur tetrafluoride is a chemical compound with the formula SF4. It is a colorless corrosive gas that releases dangerous hydrogen fluoride gas upon exposure to water or moisture. Sulfur tetrafluoride is a useful reagent for the preparation of organofluorine compounds,[3] some of which are important in the pharmaceutical and specialty chemical industries.
- ^ a b c d NIOSH Pocket Guide to Chemical Hazards. "#0580". National Institute for Occupational Safety and Health (NIOSH).
- ^ Tolles, W. M.; W. M. Gwinn, W. D. (1962). "Structure and Dipole Moment for SF4". J. Chem. Phys. 36 (5): 1119–1121. Bibcode:1962JChPh..36.1119T. doi:10.1063/1.1732702.
- ^ Wang, C.-L. J. (2004). "Sulfur Tetrafluoride". In Paquette, L. (ed.). Encyclopedia of Reagents for Organic Synthesis. New York: J. Wiley & Sons. doi:10.1002/047084289X. hdl:10261/236866. ISBN 9780471936237.


