| |
| Names | |
|---|---|
| IUPAC names
Tantalum(V) chloride
Tantalum pentachloride | |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.028.869 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| TaCl5 | |
| Molar mass | 358.213 g/mol |
| Appearance | white monoclinic crystals[1] |
| Density | 3.68 g/cm3 |
| Melting point | 216 °C (421 °F; 489 K) |
| Boiling point | 239.4 °C (462.9 °F; 512.5 K) (decomposes) |
| reacts | |
| Solubility | chloroform, CCl4 |
| +140.0×10−6 cm3/mol | |
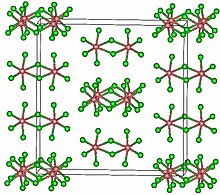
| Structure | |
| Monoclinic, mS72 | |
| C2/m, No. 12 | |
| Thermochemistry | |
Std molar
entropy (S⦵298) |
221.75 J K−1 mol−1 |
Std enthalpy of
formation (ΔfH⦵298) |
-858.98 kJ/mol |
| Hazards | |
| GHS labelling: | |
 
| |
| Danger | |
| H302, H314 | |
| P280, P305+P351+P338, P310 | |
| NFPA 704 (fire diamond) | |
| Flash point | Non-flammable |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
1900 mg/kg (oral, rat) |
| Safety data sheet (SDS) | External SDS |
| Related compounds | |
Other anions
|
Tantalum(V) fluoride Tantalum(V) bromide Tantalum(V) iodide |
Other cations
|
Vanadium(IV) chloride Niobium(V) chloride |
Related compounds
|
Tantalum(III) chloride, Tantalum(IV) chloride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Tantalum(V) chloride, also known as tantalum pentachloride, is an inorganic compound with the formula TaCl5. It takes the form of a white powder and is commonly used as a starting material in tantalum chemistry. It readily hydrolyzes to form tantalum(V) oxychloride (TaOCl3) and eventually tantalum pentoxide (Ta2O5); this requires that it be synthesised and manipulated under anhydrous conditions, using air-free techniques.
- ^ Lide, David R., ed. (2006). CRC Handbook of Chemistry and Physics (87th ed.). Boca Raton, FL: CRC Press. ISBN 0-8493-0487-3.
