| |||
| Names | |||
|---|---|---|---|
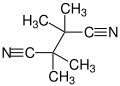
| Preferred IUPAC name
Tetramethylbutanedinitrile | |||
| Other names
Butanedinitrile, 2,2,3,3-tetramethyl [2]
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| Abbreviations | TMSN[3] | ||
| ChemSpider | |||
| ECHA InfoCard | 100.129.378 | ||
| MeSH | tetramethylsuccinonitrile | ||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C8H12N2 | |||
| Molar mass | 136.198 g·mol−1 | ||
| Appearance | Colourless crystals | ||
| Odor | odorless[4] | ||
| Density | 1.07 g mL−1 | ||
| Melting point | 169.1 °C; 336.3 °F; 442.2 K | ||
| Boiling point | sublimes[4] | ||
| Thermochemistry | |||
Std enthalpy of
formation (ΔfH⦵298) |
13.6–16.2 kJ mol−1 | ||
Std enthalpy of
combustion (ΔcH⦵298) |
−4.8767–−4.8793 MJ mol−1 | ||
| Hazards | |||
| GHS labelling: | |||
  
| |||
| Danger | |||
| H300, H310, H315, H319, H330, H370, H372, H412 | |||
| P260, P262, P264, P270, P271, P273, P280, P284, P301+P310, P302+P350, P302+P352, P304+P340, P305+P351+P338, P307+P311, P310, P314, P320, P321, P322, P330, P332+P313, P337+P313, P361, P362, P363, P403+P233, P405, P501 | |||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose)
|
38.9 mg/kg (rat, oral)[5] | ||
LCLo (lowest published)
|
28 ppm (mouse, 3 hr) 6 ppm (rat, 30 hr)[5] | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible)
|
TWA 3 mg/m3 (0.5 ppm) [skin][4] | ||
REL (Recommended)
|
TWA 3 mg/m3 (0.5 ppm) [skin][4] | ||
IDLH (Immediate danger)
|
5 ppm[4] | ||
| Safety data sheet (SDS) | ICSC 1121 | ||
| Related compounds | |||
Related alkanenitriles
|
|||
Related compounds
|
DBNPA | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Tetramethylsuccinonitrile or TMSN is an organic compound with the formula (C(CH3)2CN)2, classified as a dinitrile. It is a colorless and odorless solid. TMSN is the byproduct from the use of some radical initiators used in polymer manufacture.[6]
TMSN is derived from 2,2'-azobis-isobutyronitrile:
- (NC(CH3)2CN)2 → (C(CH3)2CN)2 + N2
AIBN is a common radical initiator in the manufacture of polyvinyl chloride polymers.
- ^ "TETRAMETHYL SUCCINONITRILE". International Chemistry Safety Cards. ILO and WHO. Retrieved 27 November 2023.
- ^ "Tetramethyl succinonitrile". Royal Society of Chemistry. Retrieved 21 October 2015.
- ^ "Tetramethyl succinonitrile". NIOSH Pocket Guide to Chemical Hazards. Centers for Disease Control and Prevention. Retrieved 15 November 2013.
- ^ a b c d e NIOSH Pocket Guide to Chemical Hazards. "#0604". National Institute for Occupational Safety and Health (NIOSH).
- ^ a b "Tetramethyl succinonitrile". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- ^ Polymeric Materials Encyclopedia, Joseph C. Salamone, 1996, CRC Press, ISBN 0-8493-2470-X