 | |
| Clinical data | |
|---|---|
| Pronunciation | /θəˈlɪdəmaɪd/[1] |
| Trade names | Contergan, Thalomid, others |
| Other names | α-Phthalimidoglutarimide |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a699032 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 90% |
| Protein binding | 55% and 66% for the (R)-(+)- and (S)-(−)-enantiomers, respectively[5] |
| Metabolism | Liver (minimally via CYP2C19-mediated 5-hydroxylation; mostly via non-enzymatic hydrolysis at the four amide sites)[5] |
| Elimination half-life | 5–7.5 hours (dose-dependent)[5] |
| Excretion | Urine, feces and semen[5] |
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.029 |
| Chemical and physical data | |
| Formula | C13H10N2O4 |
| Molar mass | 258.233 g·mol−1 |
| 3D model (JSmol) | |
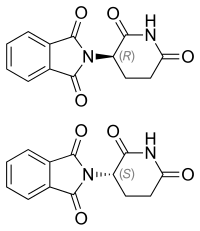
| Chirality | Racemic mixture |
| |
| |
| | |
Thalidomide, sold under the brand names Contergan and Thalomid among others, is an oral medication used to treat a number of cancers (e.g., multiple myeloma), graft-versus-host disease, and many skin disorders (e.g., complications of leprosy such as skin lesions).[6][7] Thalidomide has been used to treat conditions associated with HIV: aphthous ulcers, HIV-associated wasting syndrome, diarrhea, and Kaposi's sarcoma, but increases in HIV viral load have been reported.[6]
Common side effects include sleepiness, rash, and dizziness.[6] Severe side effects include tumor lysis syndrome, blood clots, and peripheral neuropathy.[8] Thalidomide is a known human teratogen and carries an extremely high risk of severe, life-threatening birth defects if administered during pregnancy. It causes skeletal deformities such as amelia (absence of legs and/or arms), absence of bones, and phocomelia (malformation of the limbs). A single dose of thalidomide, regardless of dosage, is enough to cause teratogenic effects.[6]
Thalidomide was first marketed in 1957 in West Germany, where it was available over the counter.[9][10] When first released, thalidomide was promoted for anxiety, trouble sleeping, "tension", and morning sickness.[10][11] While it was initially thought to be safe in pregnancy, concerns regarding birth defects arose, resulting in its removal from the market in Europe in 1961.[9][10] The total number of infants severely harmed by thalidomide use during pregnancy is estimated at over 10,000, possibly 20,000, of whom about 40% died around the time of birth.[6][10] Those who survived had limb, eye, urinary tract, and heart problems.[9] Its initial entry into the US market was prevented by Frances Kelsey, a reviewer at the FDA.[11] The birth defects caused by thalidomide led to the development of greater drug regulation and monitoring in many countries.[9][11]
It was approved in the United States in 1998 for use as a treatment for cancer.[6] It is on the World Health Organization's List of Essential Medicines.[12] It is available as a generic medication.[8][13]
- ^ "Thalidomide". Oxford English Dictionary (Online ed.). Oxford University Press. (Subscription or participating institution membership required.)
- ^ "Thalomid- thalidomide capsule". DailyMed. 11 March 2021. Archived from the original on 21 October 2022. Retrieved 21 October 2022.
- ^ "Thalidomide BMS EPAR". European Medicines Agency. 17 September 2018. Archived from the original on 21 October 2022. Retrieved 21 October 2022.
- ^ "Thalidomide Lipomed EPAR". European Medicines Agency. 18 July 2022. Archived from the original on 21 October 2022. Retrieved 21 October 2022.
- ^ a b c d Teo SK, Colburn WA, Tracewell WG, Kook KA, Stirling DI, Jaworsky MS, et al. (2004). "Clinical pharmacokinetics of thalidomide". Clinical Pharmacokinetics. 43 (5): 311–27. doi:10.2165/00003088-200443050-00004. PMID 15080764. S2CID 37728304.
- ^ a b c d e f "Thalidomide Monograph for Professionals". Drugs.com. 7 April 2023. Updated as required.
- ^ "Thalidomide | C13H10N2O4". PubChem. National Center for Biotechnology Information, National Library of Medicine. CID 5426. Archived from the original on 13 February 2023. Retrieved 13 February 2023.
- ^ a b British national formulary : BNF 76 (76 ed.). Pharmaceutical Press. 2018. p. 936. ISBN 9780857113382.
- ^ a b c d Cuthbert A (2003). The Oxford Companion to the Body. Oxford University Press. p. 682. doi:10.1093/acref/9780198524038.001.0001. ISBN 9780198524038.
- ^ a b c d Miller MT (1991). "Thalidomide embryopathy: a model for the study of congenital incomitant horizontal strabismus". Transactions of the American Ophthalmological Society. 89: 623–74. PMC 1298636. PMID 1808819.
- ^ a b c Loue S, Sajatovic M (2004). Encyclopedia of Women's Health. Springer Science & Business Media. p. 644. ISBN 9780306480737. Archived from the original on 15 November 2021. Retrieved 25 August 2020.
- ^ Organization WH (2021). Model list of essential medicines: 22nd list. Geneva: World Health Organization. hdl:10665/345533. WHO/MHP/HPS/EML/2021.02.
- ^ "First Generic Drug Approvals". U.S. Food and Drug Administration. 30 May 2023. Archived from the original on 30 June 2023. Retrieved 30 June 2023.