 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| PubChem CID | |
| ChemSpider | |
| Chemical and physical data | |
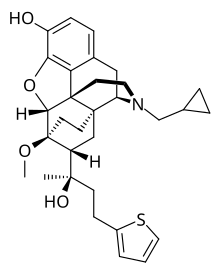
| Formula | C31H39NO4S |
| Molar mass | 521.72 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Thienorphine is a very potent, extremely long-acting, orally-active opioid analgesic with mixed agonist–antagonist properties which was developed by the Beijing Institute of Pharmacology and Toxicology as a potential treatment for opioid dependence.[1][2][3] It is a high-affinity, balanced ligand of the μ- (Ki = 0.22 nM), δ- (Ki = 0.69 nM), and κ-opioid receptors (Ki = 0.14 nM), behaving as a partial agonist of the μ- (Emax = 19%–28%) and κ-opioid receptors (Emax = 65–75%) and as an antagonist of the δ-opioid receptor.[4][5][6] It also possesses relatively low affinity for the nociceptin receptor (Ki = 36.5 nM), where it acts as an antagonist.[6]
- ^ Liu H, Zhong BH, Liu CH, Wu B, Gong ZH (2005). "Synthesis, Crystal Structural and Pharmacological Study of N-Cyclopropylmehtyl-7α-[(R)-1-hydroxyl-1-methyl-3-(thien-2-yl)propyl]-6,14-endoethanotetrahydronooripavine" (PDF). Acta Chimica Slovenica. 52 (1): 80–85. ISSN 1318-0207.
- ^ Yu G, Liu YS, Yan LD, Wen Q, Gong ZH (July 2009). "[Structure-activity relationships analysis of thienorphine and its derivatives]". Yao Xue Xue Bao [Acta Pharmaceutica Sinica] (in Chinese). 44 (7): 726–730. PMID 19806910.
- ^ Yu G, Li SH, Cui MX, Yan LD, Yong Z, Zhou PL, et al. (March 2014). "Multiple mechanisms underlying the long duration of action of thienorphine, a novel partial opioid agonist for the treatment of addiction". CNS Neuroscience & Therapeutics. 20 (3): 282–288. doi:10.1111/cns.12210. PMC 6492997. PMID 24330593.
- ^ Yu G, Yue YJ, Cui MX, Gong ZH (July 2006). "Thienorphine is a potent long-acting partial opioid agonist: a comparative study with buprenorphine". The Journal of Pharmacology and Experimental Therapeutics. 318 (1): 282–287. doi:10.1124/jpet.105.099937. PMID 16569757. S2CID 24549788.
- ^ Li JX, Becker GL, Traynor JR, Gong ZH, France CP (April 2007). "Thienorphine: receptor binding and behavioral effects in rhesus monkeys". The Journal of Pharmacology and Experimental Therapeutics. 321 (1): 227–236. doi:10.1124/jpet.106.113290. PMID 17220427. S2CID 11477535.
- ^ a b Wen Q, Yu G, Li YL, Yan LD, Gong ZH (October 2011). "Pharmacological mechanisms underlying the antinociceptive and tolerance effects of the 6,14-bridged oripavine compound 030418". Acta Pharmacologica Sinica. 32 (10): 1215–1224. doi:10.1038/aps.2011.83. PMC 4010084. PMID 21863064.