| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC names | |||
| Systematic IUPAC name
hydroxidooxidosulfanidosulfur[1] | |||
| Other names
sulfurothionous acid
Thiothionyl hydroxide (minor tautomer) | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChemSpider | |||
| 184467 | |||
PubChem CID
|
|||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| H2S2O2 | |||
| Molar mass | 98.14668 | ||
| Conjugate base | Thiosulfite | ||
| Related compounds | |||
Related compounds
|
thiosulfuric acid SSO thiosulfinic acid | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
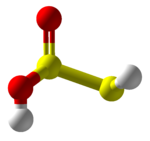
Thiosulfurous acid is a hypothetical chemical compound with the formula HS−S(=O)−OH or HO−S(=S)−OH. Attempted synthesis leads to polymers.[3] It is a low oxidation state (+1) sulfur acid.[4] It is the Arrhenius acid for disulfur monoxide. Salts derived from thiosulfurous acid, which are also unknown, are named "thiosulfites", "thionosulfites" or "sulfurothioites". The ion is S=SO2−
2.
- ^ a b International Union of Pure and Applied Chemistry (2005). Nomenclature of Inorganic Chemistry (IUPAC Recommendations 2005). Cambridge (UK): RSC–IUPAC. ISBN 0-85404-438-8. p. 139. Electronic version.
- ^ ACD Chemsketch Name Free
- ^ Schmidt, Heinar; Ralf Steudel; Detlev Suelzle; Helmut Schwarz (1992). "Sulfur compounds. 148. Generation and characterization of dihydroxy disulfide, HOSSOH: the chainlike isomer of thiosulfurous acid". Inorganic Chemistry. 31 (6): 941–944. doi:10.1021/ic00032a004. ISSN 0020-1669.
- ^ +1 is the average oxidation state of the two structurally different sulfur atoms. The exterior atom has an oxidation number of −1 while the central sulfur atom has the oxidation state of +3.