 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Stablon, Coaxil, Tatinol |
| Other names | Tia;[1] ZaZa;[2] S-1574;[3][4][5] JNJ-39823277; TPI-1062[6] |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 99%[9][10] |
| Protein binding | 95%[10] |
| Metabolism | Hepatic[10] by β-oxidation[12] |
| Elimination half-life | 2.5–3 hours[9][10] 4–9 hours (elderly)[10][11] |
| Excretion | Urine: 65%[9] Feces: 15%[10] |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.131.750 100.069.844, 100.131.750 |
| Chemical and physical data | |
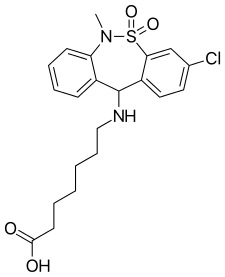
| Formula | C21H25ClN2O4S |
| Molar mass | 436.95 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Tianeptine, sold under the brand names Stablon, Tatinol, and Coaxil among others, is an atypical tricyclic antidepressant which is used mainly in the treatment of major depressive disorder, although it may also be used to treat anxiety, asthma, and irritable bowel syndrome.[3][4][5]
Tianeptine has antidepressant and anxiolytic effects[13] with a relative lack of sedative, anticholinergic, and cardiovascular side effects.[10][14] It has been found to act as an atypical agonist of the μ-opioid receptor with clinically negligible effects on the δ- and κ-opioid receptors.[15][16][17] This may explain part of its antidepressant and anxiolytic effects; however, it is thought that tianeptine also modulates glutamate receptors, and this may also explain tianeptine's antidepressant/anxiolytic effects.
Tianeptine was discovered and patented by the French Society of Medical Research in the 1960s. It was introduced for medical use in France in 1983.[18] Currently, tianeptine is approved in France and manufactured and marketed by Laboratories Servier SA; it is also marketed in a number of other European countries under the trade name Coaxil as well as in Asia (including Singapore) and Latin America as Stablon and Tatinol but it is not available in Australia, Canada, New Zealand, or the United Kingdom.[19][20] In the US, it is an unregulated drug sold under several names and some of these products have been found to be adulterated with other recreational drugs. It is commonly known by the nickname 'gas station heroin'.[21][22]
- ^ Uzbekov M (2011). "FC13-09 - Antidepressant tianeptine (TIA) action is based on the acceleration of serotonin turnover in the synapse: a hypothesis". European Psychiatry. 26: 1890. doi:10.1016/S0924-9338(11)73594-5. S2CID 143885547. Retrieved 3 December 2023.
- ^ Wagner ML, Pergolizzi J, LeQuang JA, Breve F, Varrassi G (June 2023). "From Antidepressant Tianeptine to Street Drug ZaZa: A Narrative Review". Cureus. 15 (6): e40688. doi:10.7759/cureus.40688. PMC 10359047. PMID 37485121.
- ^ a b Elks J (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 1195–. ISBN 978-1-4757-2085-3.
- ^ a b Index Nominum 2000: International Drug Directory. Taylor & Francis. 2000. pp. 1024–. ISBN 978-3-88763-075-1.
- ^ a b "Tianeptine (International database)". Drugs.com.
- ^ Cite error: The named reference
AdisInsightwas invoked but never defined (see the help page). - ^ Anvisa (31 March 2023). "RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 4 April 2023). Archived from the original on 3 August 2023. Retrieved 16 August 2023.
- ^ "Poisons Standard June 2017". 29 May 2017.
- ^ a b c Royer RJ, Albin H, Barrucand D, Salvadori-Failler C, Kamoun A (1988). "Pharmacokinetic and metabolic parameters of tianeptine in healthy volunteers and in populations with risk factors". Clinical Neuropharmacology. 11 (Suppl 2): S90-6. PMID 3180120.
- ^ a b c d e f g Wagstaff AJ, Ormrod D, Spencer CM (March 2001). "Tianeptine: a review of its use in depressive disorders". CNS Drugs. 15 (3): 231–59. doi:10.2165/00023210-200115030-00006. PMID 11463130. S2CID 37796160.
- ^ Carlhant D, Le Garrec J, Guedes Y, Salvadori C, Mottier D, Riche C (September 1990). "Pharmacokinetics and bioavailability of tianeptine in the elderly". Drug Investigation. 2 (3): 167–172. doi:10.1007/BF03259191. S2CID 56502717.
- ^ Fromenty B, Freneaux E, Labbe G, Deschamps D, Larrey D, Letteron P, et al. (1 November 1989). "Tianeptine, a new tricyclic antidepressant metabolized by beta-oxidation of its heptanoic side chain, inhibits the mitochondrial oxidation of medium and short chain fatty acids in mice". Biochem Pharmacol. 38 (21): 3743–3751. doi:10.1016/0006-2952(89)90580-7. PMID 2597170.
- ^ Defrance R, Marey C, Kamoun A (1988). "Antidepressant and anxiolytic activities of tianeptine: an overview of clinical trials" (PDF). Clinical Neuropharmacology. 11 (Suppl 2): S74-82. PMID 2902922. Archived from the original (PDF) on 4 April 2016.
- ^ Cite error: The named reference
CNS2008was invoked but never defined (see the help page). - ^ Cite error: The named reference
pmid25026323was invoked but never defined (see the help page). - ^ Berridge KC, Kringelbach ML (August 2008). "Affective neuroscience of pleasure: reward in humans and animals". Psychopharmacology. 199 (3): 457–80. doi:10.1007/s00213-008-1099-6. PMC 3004012. PMID 18311558.
- ^ El Zahran T, Schier J, Glidden E, Kieszak S, Law R, Bottei E, et al. (August 2018). "Characteristics of Tianeptine Exposures Reported to the National Poison Data System - United States, 2000-2017". MMWR. Morbidity and Mortality Weekly Report. 67 (30): 815–818. doi:10.15585/mmwr.mm6730a2. PMC 6072055. PMID 30070980.
- ^ Publishing W (2013). Pharmaceutical Manufacturing Encyclopedia. Volumes 1-4. William Andrew. p. 3222. ISBN 978-0-8155-1856-3. Retrieved 10 October 2024.
- ^ Cite error: The named reference
akikiwas invoked but never defined (see the help page). - ^ "Tianeptine Sodium". Martindale: The Complete Drug Reference. London, UK: Pharmaceutical Press. 5 December 2011. Retrieved 2 December 2013.
- ^ Cite error: The named reference
:0was invoked but never defined (see the help page). - ^ Counts CJ, Spadaro AV, Cerbini TA, Krotulski AJ, Greller HA, Nelson LS, et al. (February 2024). "Notes from the Field: Cluster of Severe Illness from Neptune's Fix Tianeptine Linked to Synthetic Cannabinoids - New Jersey, June-November 2023". MMWR. Morbidity and Mortality Weekly Report. 73 (4): 89–90. doi:10.15585/mmwr.mm7304a5. PMC 10843069. PMID 38300852.