 | |
| Clinical data | |
|---|---|
| Trade names | Travatan, Izba, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a602027 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | Topical eye drops |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Metabolism | Activation by ester hydrolysis, deactivation by beta oxidation, OH-oxidation, double bond reduction |
| Onset of action | 2 hours |
| Elimination half-life | 1.5 hours (in aqueous fluid) 45 minutes (terminal) |
| Duration of action | ≥ 24 hours |
| Excretion | Mainly via kidney |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.207.141 |
| Chemical and physical data | |
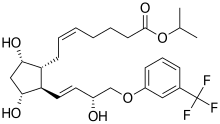
| Formula | C26H35F3O6 |
| Molar mass | 500.555 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Travoprost, sold under the brand name Travatan among others, is a medication used to treat high pressure inside the eye including glaucoma.[4] Specifically it is used for open angle glaucoma when other agents are not sufficient.[5][4] It is used as an eye drop.[4] Effects generally occur within two hours.[4]
Common side effects include red eyes, blurry vision, eye pain, dry eyes, and change in color of the eyes.[4][5] Other significant side effects may include cataracts.[5] Use during pregnancy or breastfeeding is generally not recommended.[5] It is a prostaglandin analog and works by increasing the outflow of aqueous fluid from the eyes.[4]
Travoprost was approved for medical use in the United States and in the European Union in 2001.[4][3] It is available as a generic medication in the United Kingdom.[5] In 2020, it was the 304th most commonly prescribed medication in the United States, with more than 1 million prescriptions.[6][7]
- ^ "Travoprost ophthalmic Use During Pregnancy". Drugs.com. 8 October 2019. Retrieved 16 May 2020.
- ^ "iDose TR- travoprost intracameral implant". DailyMed. 20 December 2023. Retrieved 26 February 2024.
- ^ a b "Travatan EPAR". European Medicines Agency (EMA). Retrieved 3 January 2021.
- ^ a b c d e f g "Travoprost Monograph for Professionals". Drugs.com. American Society of Health-System Pharmacists. Retrieved 26 March 2019.
- ^ a b c d e British national formulary : BNF 76 (76 ed.). Pharmaceutical Press. 2018. p. 1152. ISBN 9780857113382.
- ^ "The Top 300 of 2020". ClinCalc. Retrieved 7 October 2022.
- ^ "Travoprost - Drug Usage Statistics". ClinCalc. Retrieved 7 October 2022.