This article needs additional citations for verification. (October 2021) |
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Remodulin, Orenitram, Tyvaso, others |
| AHFS/Drugs.com | Monograph |
| License data |
|
| Pregnancy category |
|
| Routes of administration | Subcutaneous, intravenous, inhalation, by mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | ~100% |
| Metabolism | Substantially metabolized by the liver |
| Elimination half-life | 4 hours |
| Excretion | Urine (79% of administered dose is excreted as 4% unchanged drug and 64% as identified metabolites); feces (13%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.236.149 |
| Chemical and physical data | |
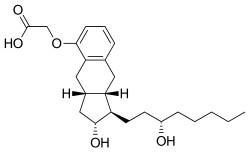
| Formula | C23H34O5 |
| Molar mass | 390.520 g·mol−1 |
| |
| | |
Treprostinil, sold under the brand names Remodulin for infusion, Orenitram for oral, and Tyvaso for inhalation, is a vasodilator that is used for the treatment of pulmonary arterial hypertension.[6]
Treprostinil was approved for use in the United States in May 2002.[7]
- ^ "Remodulin- treprostinil injection, solution; Sterile diluent for remodulin- water injection, solution". DailyMed. 9 October 2023. Retrieved 21 May 2024.
- ^ "Orenitram- treprostinil tablet, extended release; Orenitram- treprostinil kit". DailyMed. 7 November 2023. Retrieved 21 May 2024.
- ^ "Tyvaso- treprostinil inhalant". DailyMed. 8 December 2023. Retrieved 21 May 2024.
- ^ "Tyvaso DPI- treprostinil inhalant; Tyvaso DPI- treprostinil kit". DailyMed. 26 January 2024. Retrieved 21 May 2024.
- ^ Cite error: The named reference
Trepulmix EPARwas invoked but never defined (see the help page). - ^ Torres F, Rubin LJ (January 2013). "Treprostinil for the treatment of pulmonary arterial hypertension". Expert Review of Cardiovascular Therapy. 11 (1): 13–25. doi:10.1586/erc.12.160. PMID 23259441. S2CID 29661141.
- ^ "Drug Approval Package: Remodulin (Treprostinil Sodium) NDA #021272". U.S. Food and Drug Administration (FDA). 24 December 1999. Retrieved 9 April 2020.