| |

| |
| Names | |
|---|---|
| IUPAC name
Trimethylalumane
| |
| Other names
Trimethylaluminum; aluminium trimethyl; aluminum trimethyl
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.000.776 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| Properties | |
| C6H18Al2 | |
| Molar mass | 144.17 g/mol 72.09 g/mol (C3H9Al) |
| Appearance | Colorless liquid |
| Density | 0.752 g/cm3 |
| Melting point | 15 °C (59 °F; 288 K) |
| Boiling point | 125–130 °C (257–266 °F; 398–403 K)[1][2] |
| Reacts | |
| Vapor pressure |
|
| Viscosity |
|
| Thermochemistry | |
Heat capacity (C)
|
155.6 J/mol·K[2] |
Std molar
entropy (S⦵298) |
209.4 J/mol·K[2] |
Std enthalpy of
formation (ΔfH⦵298) |
−136.4 kJ/mol[2] |
Gibbs free energy (ΔfG⦵)
|
−9.9 kJ/mol[2] |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Pyrophoric |
| GHS labelling: | |
  [1] [1]
| |
| Danger | |
| H250, H260, H314[1] | |
| P222, P223, P231+P232, P280, P370+P378, P422[1] | |
| NFPA 704 (fire diamond) | |
| Flash point | −17.0 °C (1.4 °F; 256.1 K)[1] |
| Related compounds | |
Related compounds
|
Triethylaluminium |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
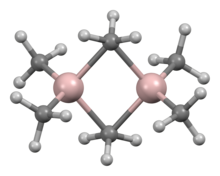
Trimethylaluminium is one of the simplest examples of an organoaluminium compound. Despite its name it has the formula Al2(CH3)6 (abbreviated as Al2Me6 or TMA), as it exists as a dimer. This colorless liquid is pyrophoric. It is an industrially important compound, closely related to triethylaluminium.[3][4]
- ^ a b c d e f Sigma-Aldrich Co., Trimethylaluminum. Retrieved on 2014-05-05.
- ^ a b c d e "Trimethyl aluminum".
- ^ Krause, Michael J.; Orlandi, Frank; Saurage, Alfred T.; Zietz, Joseph R. (2000). "Aluminum Compounds, Organic". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a01_543. ISBN 978-3527306732.
- ^ C. Elschenbroich (2006). Organometallics. VCH. ISBN 978-3-527-29390-2.
