 | |
| Clinical data | |
|---|---|
| Trade names | different brandnames, typical example: "TMCP-018", "KM-X1", "UR-144", "MN-001", "YX-17" |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
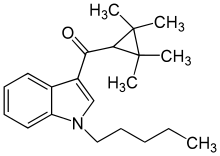
| Formula | C21H29NO |
| Molar mass | 311.469 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
UR-144 (TMCP-018, KM-X1, MN-001, YX-17) is a drug invented by Abbott Laboratories,[2] that acts as a selective full agonist of the peripheral cannabinoid receptor CB2, but with much lower affinity for the psychoactive CB1 receptor.
- ^ Anvisa (2023-07-24). "RDC Nº 804 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 804 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 2023-07-25). Archived from the original on 2023-08-27. Retrieved 2023-08-27.
- ^ WO application 2006069196, Pace JM, Tietje K, Dart MJ, Meyer MD, "3-Cycloalkylcarbonyl indoles as cannabinoid receptor ligands", published 2006-06-29, assigned to Abbott Laboratories