You can help expand this article with text translated from the corresponding article in Russian. (June 2020) Click [show] for important translation instructions.
|
This article needs more reliable medical references for verification or relies too heavily on primary sources. (July 2020) |  |
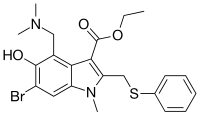 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Arbidol |
| Other names | AR-1I9514, Russian: Арбидол, Chinese: 阿比朵尔.[citation needed] |
| Pregnancy category |
|
| Routes of administration | By mouth (hard capsules, tablets) |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 40% |
| Metabolism | Hepatic, CYP3A4[3][4] |
| Elimination half-life | 17–21 hours[3][4] |
| Excretion | 40% excrete as unchanged umifenovir in feces (38.9%) and urine (0.12%)[5] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.247.800 |
| Chemical and physical data | |
| Formula | C22H25BrN2O3S |
| Molar mass | 477.42 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Umifenovir, sold under the brand name Arbidol, is sold and used as an antiviral medication for influenza in Russia and China. The drug is manufactured by Pharmstandard (Russian: Фармстандарт). It is not approved by the U.S. Food and Drug Administration (FDA) for the treatment or prevention of influenza.[6]
- ^ a b "阿比朵尔抑制新冠,先声药业生产的吗?". 全民健康网 (in Chinese). Archived from the original on 17 June 2020. Retrieved 17 June 2020.
- ^ "ИНСТРУКЦИЯ ПО ПРИМЕНЕНИЮ АРПЕТОЛ (ARPETOL)". Vidal (in Belarusian). Archived from the original on 26 January 2018. Retrieved 17 June 2020.
- ^ a b Cite error: The named reference
pharmk1was invoked but never defined (see the help page). - ^ a b Cite error: The named reference
pharmk2was invoked but never defined (see the help page). - ^ "Full Prescribing Information: Arbidol (umifenovir) film-coated tablets 50 and 100 mg: Corrections and Additions". State Register of Medicines (in Russian). Open joint-stock company "Pharmstandard-Tomskchempharm". Retrieved 3 June 2015.[permanent dead link]
- ^ "FDA Approved Drugs for Influenza". U.S. Food and Drug Administration. 8 December 2022.