| |
| Names | |
|---|---|
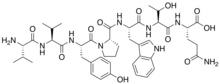
| IUPAC name
L-Valyl-L-valyl-L-tyrosyl-L-prolyl-L-tryptophyl-L-threonyl-L-glutamine
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C44H61N9O11 | |
| Molar mass | 892.024 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Valorphin, also known as VV-hemorphin-5, is a naturally occurring, endogenous opioid heptapeptide of the hemorphin family with the amino acid sequence H-Val-Val-Tyr-Pro-Trp-Thr-Gln-OH (VVYPWTQ).[1][2][3] It is produced in the body via proteolyic cleavage of residues 33-39 of the β-chain of hemoglobin.[2][4] Valorphin binds preferentially to the μ-opioid receptor and produces effects such as analgesia and self-administration in animals.[1][2] It also possesses cytotoxic and antiproliferative properties against tumor cells,[3][4][5][6] the mediation of which, because they are reversed by naloxone, appears to be dependent on the opioid receptors.[5]
- ^ a b Maurer R, Römer D, Büscher HH, Gähwiler BH, Thies PW, David S (February 1985). "Valorphin: a novel chemical structure with opioid activity". Neuropeptides. 5 (4–6): 387–90. doi:10.1016/0143-4179(85)90035-6. PMID 2860596. S2CID 46078832.
- ^ a b c Erchegyi J, Kastin AJ, Zadina JE, Qiu XD (June 1992). "Isolation of a heptapeptide Val-Val-Tyr-Pro-Trp-Thr-Gln (valorphin) with some opiate activity". International Journal of Peptide and Protein Research. 39 (6): 477–84. doi:10.1111/j.1399-3011.1992.tb00277.x. PMID 1356941.
- ^ a b Blishchenko EY, Sazonova OV, Kalinina OA, et al. (May 2002). "Family of hemorphins: co-relations between amino acid sequences and effects in cell cultures". Peptides. 23 (5): 903–10. doi:10.1016/S0196-9781(02)00017-7. PMID 12084521. S2CID 44834813.
- ^ a b Blishchenko E, Sazonova O, Surovoy A, et al. (August 2002). "Antiproliferative action of valorphin in cell cultures". Journal of Peptide Science. 8 (8): 438–52. doi:10.1002/psc.402. PMID 12212807. S2CID 46177846.
- ^ a b Blishchenko EYu; Mernenko OA; Mirkina II; et al. (1997). "Tumor cell cytolysis mediated by valorphin, an opioid-like fragment of hemoglobin beta-chain". Peptides. 18 (1): 79–85. doi:10.1016/S0196-9781(96)00248-3. PMID 9114456. S2CID 11332853.
- ^ Blishchenko EY, Sazonova OV, Kalinina OA, et al. (January 2005). "Antitumor effect of valorphin in vitro and in vivo: combined action with cytostatic drugs". Cancer Biology & Therapy. 4 (1): 118–24. doi:10.4161/cbt.4.1.1474. PMID 15662114.