| |
 Xylitol crystals
| |
| Names | |
|---|---|
| Pronunciation | /ˈzaɪlɪtɒl/ |
| IUPAC name
meso-Xylitol
| |
| Systematic IUPAC name
(2R,3R,4S)-Pentane-1,2,3,4,5-pentol | |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.001.626 |
| E number | E967 (glazing agents, ...) |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C5H12O5 | |
| Molar mass | 152.146 g·mol−1 |
| Density | 1.52 g/cm3 |
| Melting point | 92 to 96 °C (198 to 205 °F; 365 to 369 K) |
| Boiling point | 345.39 °C (653.70 °F; 618.54 K) Predicted value using Adapted Stein & Brown method[2] |
| ~100 g/L | |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Related compounds | |
Related alkanes
|
Pentane |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
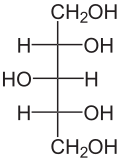
Xylitol is a chemical compound with the formula C
5H
12O
5, or HO(CH2)(CHOH)3(CH2)OH; specifically, one particular stereoisomer with that structural formula. It is a colorless or white crystalline solid that is freely soluble in water. It is classified as a polyalcohol and a sugar alcohol, specifically an alditol. The name derives from Ancient Greek: ξύλον, xyl[on] 'wood', with the suffix -itol used to denote it being a sugar alcohol.
Xylitol is used as a food additive and sugar substitute. Its European Union code number is E967.[3] Replacing sugar with xylitol in food products may promote better dental health, but evidence is lacking on whether xylitol itself prevents dental cavities.[4][5] In the United States, xylitol is used as a common sugar substitute, and is considered to be safe.[6]
- ^ Safety data sheet for xylitol Archived 3 March 2016 at the Wayback Machine from Fisher Scientific. Retrieved 2014-11-02.
- ^ "Xylitol". Chemspider.com. Chemical Structure. Retrieved 13 May 2015.
- ^ "Food legislation". polyols-eu.org. European Association of Polyol Producers. 22 March 2017. Retrieved 7 February 2019.
- ^ Riley, P.; Moore, D.; Ahmed, F.; Sharif, M.O.; Worthington, H.V. (26 March 2015). "Xylitol-containing products for preventing dental caries in children and adults". The Cochrane Database of Systematic Reviews. 2015 (3): CD010743. doi:10.1002/14651858.CD010743.pub2. PMC 9345289. PMID 25809586.
- ^ Riley, P.; Moore, D.; Ahmed, F.; Sharif, M. O.; Worthington, H. V. (2015). "Can xylitol – used in products like sweets, candy, chewing gum, and toothpaste – help prevent tooth decay in children and adults?". The Cochrane Database of Systematic Reviews. Lay summary. 2015 (3): CD010743. doi:10.1002/14651858.CD010743.pub2. PMC 9345289. PMID 25809586.

- ^ "Aspartame and Other Sweeteners in Food". US Food and Drug Administration. 14 July 2023. Retrieved 12 September 2024.
