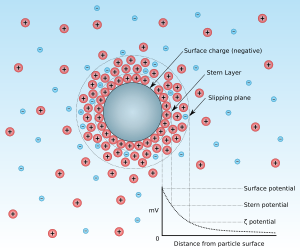
Zeta potential is the electrical potential at the slipping plane. This plane is the interface which separates mobile fluid from fluid that remains attached to the surface.
Zeta potential is a scientific term for electrokinetic potential[1][2] in colloidal dispersions. In the colloidal chemistry literature, it is usually denoted using the Greek letter zeta (ζ), hence ζ-potential. The usual units are volts (V) or, more commonly, millivolts (mV). From a theoretical viewpoint, the zeta potential is the electric potential in the interfacial double layer (DL) at the location of the slipping plane relative to a point in the bulk fluid away from the interface. In other words, zeta potential is the potential difference between the dispersion medium and the stationary layer of fluid attached to the dispersed particle.
The zeta potential is caused by the net electrical charge contained within the region bounded by the slipping plane, and also depends on the location of that plane. Thus, it is widely used for quantification of the magnitude of the charge. However, zeta potential is not equal to the Stern potential or electric surface potential in the double layer,[3][4][5][6] because these are defined at different locations. Such assumptions of equality should be applied with caution. Nevertheless, zeta potential is often the only available path for characterization of double-layer properties.
The zeta potential is an important and readily measurable indicator of the stability of colloidal dispersions. The magnitude of the zeta potential indicates the degree of electrostatic repulsion between adjacent, similarly charged particles in a dispersion. For molecules and particles that are small enough, a high zeta potential will confer stability, i.e., the solution or dispersion will resist aggregation. When the potential is small, attractive forces may exceed this repulsion and the dispersion may break and flocculate. So, colloids with high zeta potential (negative or positive) are electrically stabilized while colloids with low zeta potentials tend to coagulate or flocculate as outlined in the table.[7]

Zeta potential can also be used for the pKa estimation of complex polymers that is otherwise difficult to measure accurately using conventional methods. This can help studying the ionisation behaviour of various synthetic and natural polymers under various conditions and can help in establishing standardised dissolution-pH thresholds for pH responsive polymers.[8]
| Magnitude of Zeta potential (mV) | Stability behavior |
|---|---|
| 0 to 5 | Rapid coagulation or flocculation |
| 10 to 30 | Incipient instability |
| 30 to 40 | Moderate stability |
| 40 to 60 | Good stability |
| >61 | Excellent stability |
- ^ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "electrokinetic potential, ζ". doi:10.1351/goldbook.E01968
- ^ "Colloidal systems – Methods for Zeta potential determination". ISO International Standard 13099, Parts 1,2 and 3. International Organization for Standardization (ISO). 2012.
- ^ Cite error: The named reference
Lyklema1995was invoked but never defined (see the help page). - ^ Cite error: The named reference
russel1992was invoked but never defined (see the help page). - ^ Cite error: The named reference
Dukhinwas invoked but never defined (see the help page). - ^ Kirby BJ (2010). Micro- and Nanoscale Fluid Mechanics: Transport in Microfluidic Devices. Cambridge University Press. ISBN 978-0-521-11903-0.[page needed]
- ^ Hanaor D, Michelazzi M, Leonelli C, Sorrell CC (2012). "The effects of carboxylic acids on the aqueous dispersion and electrophoretic deposition of ZrO2". Journal of the European Ceramic Society. 32 (1): 235–244. arXiv:1303.2754. doi:10.1016/j.jeurceramsoc.2011.08.015. S2CID 98812224.
- ^ a b Barbosa JA, Abdelsadig MS, Conway BR, Merchant HA (December 2019). "Using zeta potential to study the ionisation behaviour of polymers employed in modified-release dosage forms and estimating their pKa". International Journal of Pharmaceutics. 1: 100024. doi:10.1016/j.ijpx.2019.100024. PMC 6733289. PMID 31517289.
- ^ Kumar A, Dixit CK (2017). "Methods for characterization of nanoparticles". Advances in Nanomedicine for the Delivery of Therapeutic Nucleic Acids. pp. 43–58. doi:10.1016/B978-0-08-100557-6.00003-1. ISBN 978-0-08-100557-6.