| |
| Names | |
|---|---|
| Other names
Neutral zinc cyanide (1:2)
| |
| Identifiers | |
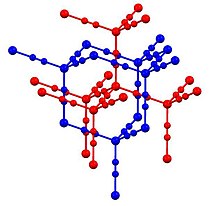
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.008.331 |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 1713 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| Zn(CN)2 | |
| Molar mass | 117.444 g/mol |
| Appearance | white solid |
| Density | 1.852 g/cm3, solid |
| Melting point | 800 °C (1,470 °F; 1,070 K) (decomposes) |
| 0.0005 g/100 mL (20 °C) | |
| Solubility | attacked by alkalies, KCN, ammonia |
| −46.0·10−6 cm3/mol | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Toxic, releases cyanide-ion in body[1] |
| GHS labelling:[2] | |
 
| |
| Danger | |
| H300, H301, H310, H330, H410 | |
| P260, P262, P264, P270, P271, P273, P280, P284, P301+P310, P302+P350, P304+P340, P310, P320, P322, P330, P361, P363, P391, P403+P233, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
100 mg/kg, rat (intraperitoneal) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Zinc cyanide is the inorganic compound with the formula Zn(CN)2. It is a white solid that is used mainly for electroplating zinc but also has more specialized applications for the synthesis of organic compounds.
- ^ Zinc cyanide toxicity
- ^ "ZINC cyanide". pubchem.ncbi.nlm.nih.gov.
- ^ "ZINC CYANIDE | CAMEO Chemicals | NOAA". cameochemicals.noaa.gov.
