 | |
| Vaccine description | |
|---|---|
| Target | Herpes zoster, postherpetic neuralgia, Ramsay Hunt syndrome type II |
| Vaccine type |
|
| Clinical data | |
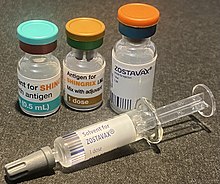
| Trade names | Zostavax, Shingrix |
| License data | |
| Pregnancy category |
|
| Routes of administration |
|
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| DrugBank | |
| ChemSpider |
|
| UNII | |
| KEGG | |
| (verify) | |
A zoster vaccine is a vaccine that reduces the incidence of herpes zoster (shingles), a disease caused by reactivation of the varicella zoster virus, which is also responsible for chickenpox.[8] Shingles provokes a painful rash with blisters, and can be followed by chronic pain (postherpetic neuralgia), as well as other complications. Older people are more often affected, as are people with weakened immune systems (immunosuppression). Both shingles and postherpetic neuralgia can be prevented by vaccination.[9]
Two zoster vaccines have been approved for use in people over 50 years old.[9] Shingrix (GSK) is a recombinant subunit vaccine which has been used in many countries since 2017.[10] Zostavax (Merck), in use since 2006,[11] is an attenuated vaccine which consists of a larger-than-normal dose of chickenpox vaccine.[8] Unlike Shingrix, Zostavax is not suitable for people with immunosuppression or diseases that affect the immune system.[9] Zostavax was discontinued in the United States in November 2020.[12]
Shingrix appears to prevent more cases of shingles than Zostavax, although side effects seem to be more frequent.[10][13]
Another vaccine, known as varicella vaccine, is used to prevent diseases caused by the same virus.[14]
- ^ "Zoster vaccine live (Zostavax) Use During Pregnancy". Drugs.com. 3 January 2020. Archived from the original on 16 May 2021. Retrieved 23 January 2020.
- ^ "Vaccines". Health Canada. 9 May 2018. Retrieved 13 April 2024.
- ^ "Zostavax vaccine – Summary of Product Characteristics (SmPC)". (emc). 28 January 2019. Archived from the original on 16 May 2021. Retrieved 6 September 2020.
- ^ Cite error: The named reference
Zostavax FDA labelwas invoked but never defined (see the help page). - ^ Cite error: The named reference
Shingrix FDA labelwas invoked but never defined (see the help page). - ^ Cite error: The named reference
Zostavax EPARwas invoked but never defined (see the help page). - ^ Cite error: The named reference
Shingrix EPARwas invoked but never defined (see the help page). - ^ a b Gershon AA, Breuer J, Cohen JI, Cohrs RJ, Gershon MD, Gilden D, et al. (July 2015). "Varicella zoster virus infection". Nature Reviews. Disease Primers. 1: 15016. doi:10.1038/nrdp.2015.16. PMC 5381807. PMID 27188665.
- ^ a b c Saguil A, Kane S, Mercado M, Lauters R (November 2017). "Herpes zoster and postherpetic neuralgia: prevention and management". American Family Physician. 96 (10): 656–663. PMID 29431387. Archived from the original on 25 April 2021. Retrieved 19 February 2021.
- ^ a b Cite error: The named reference
pmid30361202was invoked but never defined (see the help page). - ^ Pan CX, Lee MS, Nambudiri VE (2022). "Global herpes zoster incidence, burden of disease, and vaccine availability: a narrative review". Therapeutic Advances in Vaccines and Immunotherapy. 10. doi:10.1177/25151355221084535. PMC 8941701. PMID 35340552.
- ^ Cite error: The named reference
cdcwas invoked but never defined (see the help page). - ^ Gagliardi AM, Andriolo BN, Torloni MR, Soares BG, de Oliveira Gomes J, Andriolo RB, et al. (November 2019). "Vaccines for preventing herpes zoster in older adults". The Cochrane Database of Systematic Reviews. 2019 (11). doi:10.1002/14651858.CD008858.pub4. PMC 6836378. PMID 31696946.
- ^ "Herpes Zoster Vaccination". U.S. Centers for Disease Control and Prevention (CDC). 31 July 2015. Archived from the original on 26 October 2021. Retrieved 26 October 2021.