
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
Buta-1,2-diene | |
| Other names
Methyl allene
| |
| Identifiers | |
3D model (JSmol)
|
|
| 1730808 | |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.008.796 |
| EC Number |
|
| 1144 | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C4H6 | |
| Molar mass | 54.092 g·mol−1 |
| Density | 0.676 at 4 °C |
| Melting point | −136.2 °C (−213.2 °F; 137.0 K) |
| Boiling point | 10.9 °C (51.6 °F; 284.0 K) |
Refractive index (nD)
|
1.4205 at 1 °C |
| Thermochemistry | |
Enthalpy of vaporization (ΔfHvap)
|
23.426 kJ/mol |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Frostbite, flammable |
| GHS labelling: | |
 
| |
| Danger | |
| H220, H224, H411 | |
| P210, P233, P240, P241, P242, P243, P273, P280, P303+P361+P353, P370+P378, P377, P381, P391, P403, P403+P235, P410+P403, P501 | |
| Flash point | −75 °C (−103 °F; 198 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
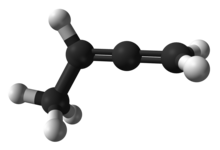
1,2-Butadiene is the organic compound with the formula CH2=C=CHCH3. It is an isomer of 1,3-butadiene, a common monomer used to make synthetic rubber. It is a colorless flammable gas, one of the simplest substituted allenes.[1]
- ^ "Product Safety Summary 1,2-BUTADIENE". ExxonMobil. March 2016.