
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
3,4-Dihydro-2H-naphthalen-1-one | |
| Other names
α-Tetralone; 1-Tetralone
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.007.692 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C10H10O | |
| Molar mass | 146.189 g·mol−1 |
| Appearance | colorless liquid |
| Density | 1.099 g·cm−3 (25 °C)[1] |
| Melting point | 2–7 °C[1] |
| Boiling point | 255–257 °C[2] 113–116 °C (8 hPa)[1] |
| insoluble[3] | |
| Solubility | soluble in organic solvents |
| Vapor pressure | 2.7 Pa (20 °C)[3] |
Refractive index (nD)
|
1.5672 |
| Hazards | |
| GHS labelling:[4] | |

| |
| Warning | |
| H302 | |
| P264, P270, P301+P317, P330, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
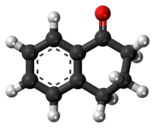
1-Tetralone is a bicyclic aromatic hydrocarbon and a ketone. In terms of its structure, it can also be regarded as benzo-fused cyclohexanone. It is a colorless oil with a faint odor.[5] It is used as starting material for agricultural and pharmaceutical agents. The carbon skeleton of 1-tetralone is found in natural products such as Aristelegone A (4,7-dimethyl-6-methoxy-1-tetralone) from the family of Aristolochiaceae used in traditional Chinese medicine.[6]
- ^ a b c Sigma-Aldrich Co., α-Tetralon. Retrieved on 25. November 2017.
- ^ William M. Haynes (2016), CRC Handbook of Chemistry and Physics, 97th Edition, Boca Raton, FL, U.S.A.: CRC Press, pp. 3–504, ISBN 978-1-4987-5429-3
- ^ a b "alpha-Tetralone 529-34-0 | TCI Deutschland GmbH". www.tcichemicals.com (in German). Retrieved 2017-12-17.
- ^ "1-Tetralone". pubchem.ncbi.nlm.nih.gov.
- ^ Cite error: The named reference
Snyderwas invoked but never defined (see the help page). - ^ P.-C. Kuo; Y.-C. Li; T.-S. Wu (2012), "Chemical constituents and pharmacology of the Aristolochia species", EJTCM, vol. 2, no. 4, pp. 249–266, doi:10.1016/S2225-4110(16)30111-0, PMC 3942903, PMID 24716140