| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Tricyclo[1.1.1.01,3]pentane | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
PubChem CID
|
|||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C5H6 | |||
| Molar mass | 66.103 g·mol−1 | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
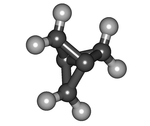
[1.1.1]Propellane is an organic compound, the simplest member of the propellane family. It is a hydrocarbon with formula C5H6 or C2(CH2)3. The molecular structure consists of three rings of three carbon atoms each, sharing one C–C bond.
[1.1.1]Propellane is a highly strained molecule. The bonds of the two central carbon atoms have an inverted tetrahedral geometry, and the length of the central bond is 160 pm. The strength of that bond is disputed; estimates vary from 59–65 kcal/mol to no strength at all. The energy of the biradical state (with no central bond at all) is calculated to be 80 kcal/mol higher. At 114 °C it will spontaneously isomerize to 3-methylidenecyclobutene (5 below) with a half-life of 5 minutes. Its strain energy is estimated to be 102 kcal/mol (427 kJ/mol). Surprisingly, [1.1.1]propellane is persistent at room temperature and is somewhat less susceptible to thermal decomposition than the less strained (90 kcal/mol) [2.2.2]propellane system, which has an estimated half-life of only about 1 h at 25 °C.[1] This unusual stability is attributed to delocalisation of electron density from the bond between the central carbon atoms onto the bridging carbon atoms.[2]
The type of bonding in this molecule has been explained in terms of charge-shift bonding.[3]
- ^ "Houben-Weyl Methods of Organic Chemistry Vol. E 17e, 4th Edition Supplement (E-Book PDF) - Thieme.de - Thieme Webshop - Armin de Meijere, Holger Butenschön, Hak-Fun Chow, Lutz Fitjer, Günter Haufe". Thieme Webshop (in German). Archived from the original on October 22, 2017. Retrieved 2017-10-21.
- ^ Sterling, Alistair J.; Dürr, Alexander; Smith, Russell; Anderson, Edward Alexander; Duarte, Fernanda (2020-04-13). "Rationalizing the diverse reactivity of [1.1.1]propellane through sigma-pi-delocalization". Chemical Science. 11 (19): 4895–4903. doi:10.1039/D0SC01386B. ISSN 2041-6539. PMC 8159217. PMID 34122945.
- ^ Wu, Wei; Gu, Junjing; Song, Jinshuai; Shaik, Sason; Hiberty, Philippe C. (2009). "The Inverted Bond in [1.1.1]Propellane is a Charge-Shift Bond". Angew. Chem. Int. Ed. 48 (8): 1407–1410. doi:10.1002/anie.200804965. PMID 19072971.