| |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
2,2-Dimethylbutane[2] | |||
| Other names
Neohexane,[1] 22DMB
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| 1730736 | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.000.825 | ||
| EC Number |
| ||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 1208 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C6H14 | |||
| Molar mass | 86.178 g·mol−1 | ||
| Appearance | Colorless liquid | ||
| Odor | Odorless | ||
| Density | 649 mg mL−1 | ||
| Melting point | −102 to −98 °C; −152 to −145 °F; 171 to 175 K | ||
| Boiling point | 49.7 to 49.9 °C; 121.4 to 121.7 °F; 322.8 to 323.0 K | ||
| log P | 3.51 | ||
| Vapor pressure | 36.88 kPa (at 20 °C) | ||
Henry's law
constant (kH) |
6.5 nmol Pa−1 kg−1 | ||
| -76.24·10−6 cm3/mol | |||
Refractive index (nD)
|
1.369 | ||
| Thermochemistry | |||
Heat capacity (C)
|
189.67 J K−1 mol−1 | ||
Std molar
entropy (S⦵298) |
272.00 J K−1 mol−1 | ||
Std enthalpy of
formation (ΔfH⦵298) |
−214.4–−212.4 kJ mol−1 | ||
Std enthalpy of
combustion (ΔcH⦵298) |
−4.1494–−4.1476 MJ mol−1 | ||
| Hazards | |||
| GHS labelling: | |||
   
| |||
| Danger | |||
| H225, H304, H315, H336, H411 | |||
| P210, P261, P273, P301+P310, P331 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | −29 °C (−20 °F; 244 K) | ||
| 425 °C (797 °F; 698 K) | |||
| Explosive limits | 1.2–7.7% | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible)
|
none[3] | ||
| Related compounds | |||
Related alkanes
|
|||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
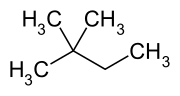
2,2-Dimethylbutane, trivially known as neohexane at William Odling's 1876 suggestion,[4] is an organic compound with formula C6H14 or (H3C-)3-C-CH2-CH3. It is therefore an alkane, indeed the most compact and branched of the hexane isomers — the only one with a quaternary carbon and a butane (C4) backbone.
- ^ Haynes, William M. (2010). Handbook of Chemistry and Physics (91 ed.). Boca Raton, Florida, USA: CRC Press. p. 3-194. ISBN 978-1-43982077-3.
- ^ "2,2-DIMETHYLBUTANE - Compound Summary". PubChem Compound. USA: National Center for Biotechnology Information. 26 March 2005. Identification and Related Records. Retrieved 9 March 2012.
- ^ NIOSH Pocket Guide to Chemical Hazards. "#0323". National Institute for Occupational Safety and Health (NIOSH).
- ^ Philosophical Magazine. Taylor & Francis. 1876.


