| |

| |
| Names | |
|---|---|
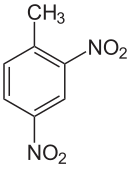
| Preferred IUPAC name
1-Methyl-2,4-dinitrobenzene | |
| Other names
Dinitrotoluol, Methyldinitrobenzene
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.004.046 |
| KEGG | |
PubChem CID
|
|
| UNII | |
| UN number | Molten: 1600 Solid or liquid: 2038 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C7H6N2O4 | |
| Molar mass | 182.134 g/mol |
| Appearance | Pale yellow to orange crystalline solid |
| Density | 1.52 g/cm3[1] |
| Melting point | 70 °C (158 °F; 343 K)[1] |
| Boiling point | Decomposes at 250–300 °C[1] |
| Vapor pressure | 1.47X10-4 mm Hg @ 22 C |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
carcinogen, combustible (though difficult to ignite)[3] |
| Flash point | 207 °C; 404 °F; 480 K |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
1,954 mg/kg (oral, mouse)[4] |
LDLo (lowest published)
|
27 mg/kg (cat, oral)[4] |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
TWA 1.5 mg/m3 [skin][3] |
REL (Recommended)
|
Ca TWA 1.5 mg/m3 [skin][3] |
IDLH (Immediate danger)
|
Ca [50 mg/m3][3] |
| Explosive data | |
| Shock sensitivity | Insensitive |
| Friction sensitivity | Very low |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
2,4-Dinitrotoluene (DNT) or dinitro is an organic compound with the formula C7H6N2O4. This pale yellow crystalline solid is well known as a precursor to trinitrotoluene (TNT) but is mainly produced as a precursor to toluene diisocyanate.
- ^ a b c Record of 2,4-Dinitrotoluene in the GESTIS Substance Database of the Institute for Occupational Safety and Health, accessed on 9. October 2007.
- ^ Pella, PA. J. Chem. Thermodyn. 9: 301-305, 1977
- ^ a b c d NIOSH Pocket Guide to Chemical Hazards. "#0235". National Institute for Occupational Safety and Health (NIOSH).
- ^ a b "Dinitrotoluene (mixed isomers)". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH). 4 December 2014. Retrieved 17 March 2015.