
2C (2C-x) is a general name for the family of psychedelic phenethylamines containing methoxy groups on the 2 and 5 positions of a benzene ring.[1] Most of these compounds also carry lipophilic substituents at the 4 position, usually resulting in more potent and more metabolically stable and longer acting compounds.[2] Most of the currently known 2C compounds were first synthesized by Alexander Shulgin in the 1970s and 1980s and published in his book PiHKAL (Phenethylamines i Have Known And Loved). Shulgin also coined the term 2C, being an acronym for the 2 carbon atoms between the benzene ring and the amino group.[3]
| Nomenclature | R3 | R4 | 2D Structure | CAS number |
|---|---|---|---|---|
| 2C-B | H | Br | 
|
66142-81-2 |
| 2C-Bn | H | CH2C6H5 | 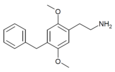
|
|
| 2C-Bu | H | CH2CH2CH2CH3 | 
|
|
| 2C-C | H | Cl | 
|
88441-14-9 |
| 2C-C-3 [4] | Cl | Cl | 
|
|
| 2C-CN | H | C≡N | 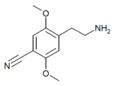
|
88441-07-0 |
| 2C-D | H | CH3 | 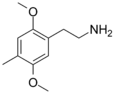
|
24333-19-5 |
| 2C-E | H | CH2CH3 | 
|
71539-34-9 |
| 2C-EF | H | CH2CH2F | 
|
1222814-77-8 |
| 2C-F | H | F | 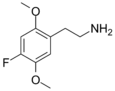
|
207740-15-6 |
| 2C-G | CH3 | CH3 | 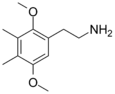
|
207740-18-9 |
| 2C-G-1 | CH2 | 
|
||
| 2C-G-2 | (CH2)2 | 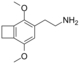
|
||
| 2C-G-3 | (CH2)3 | 
|
207740-19-0 | |
| 2C-G-4 | (CH2)4 | 
|
952006-59-6 | |
| 2C-G-5 | (CH2)5 | 
|
207740-20-3 | |
| 2C-G-6 | (CH2)6 | 
|
||
| 2C-G-N | (CH)4 | 
|
207740-21-4 | |
| 2C-H | H | H | 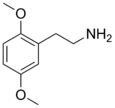
|
3600-86-0 |
| 2C-I | H | I | 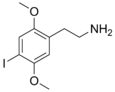
|
69587-11-7 |
| 2C-iP | H | CH(CH3)2 | 
|
1498978-47-4 |
| 2C-TBU | H | C(CH3)3 | 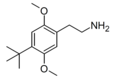
|
|
| 2C-CP | H | C3H5 | 
|
2888537-46-8 |
| 2C-CPE | H | C5H9 | 
|
|
| 2C-N | H | NO2 | 
|
261789-00-8 |
| 2C-NH2 | H | NH2 | 
|
168699-66-9 |
| 2C-PYR | H | Pyrrolidine | 
|
|
| 2C-PIP | H | Piperidine | 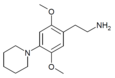
|
|
| 2C-O | H | OCH3 | 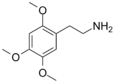
|
15394-83-9 |
| 2C-O-4 | H | OCH(CH3)2 | 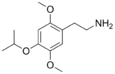
|
952006-65-4 |
| 2C-MOM [5] | H | CH2OCH3 | 
|
|
| 2C-P | H | CH2CH2CH3 | 
|
207740-22-5 |
| 2C-Ph | H | C6H5 | 
|
|
| 2C-Se | H | Se CH3 | 
|
1189246-68-1 |
| 2C-T | H | SCH3 | 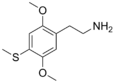
|
61638-09-3 |
| 2C-T-2 | H | SCH2CH3 | 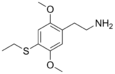
|
207740-24-7 |
| 2C-T-3[6] | H | SCH2C(=CH2)CH3 | 
|
648957-40-8 |
| 2C-T-4 | H | SCH(CH3)2 | 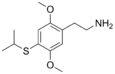
|
207740-25-8 |
| 2C-T-5[6] | 
|
|||
| 2C-T-6[6] | 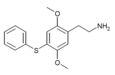
|
|||
| 2C-T-7 | H | S(CH2)2CH3 | 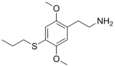
|
207740-26-9 |
| 2C-T-8 | H | SCH2CH(CH2)2 | 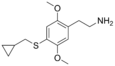
|
207740-27-0 |
| 2C-T-9[6] | 
|
207740-28-1 | ||
| 2C-T-10[6] | 
|
|||
| 2C-T-11[6] | 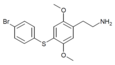
|
|||
| 2C-T-12[6] | 
|
|||
| 2C-T-13 | H | S(CH2)2OCH3 | 
|
207740-30-5 |
| 2C-T-14[6] | 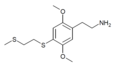
|
|||
| 2C-T-15 | H | SCH(CH2)2 | 
|
|
| 2C-T-16[7] | H | SCH2CH=CH2 | 
|
648957-42-0 |
| 2C-T-17 | H | SCH(CH3)CH2CH3 | 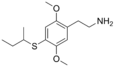
|
207740-32-7 |
| 2C-T-18[6] | 
|
|||
| 2C-T-19 | H | SCH2CH2CH2CH3 | 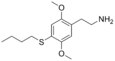
|
|
| 2C-T-21 | H | S(CH2)2F | 
|
207740-33-8 |
| 2C-T-21.5[6] | 
|
648957-46-4 | ||
| 2C-T-22[6] | 
|
648957-48-6 | ||
| 2C-T-23[6] | 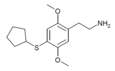
|
|||
| 2C-T-24[6] | 
|
|||
| 2C-T-25[6] | 
|
|||
| 2C-T-27[6] | 
|
648957-52-2 | ||
| 2C-T-28[6] | 
|
648957-54-4 | ||
| 2C-T-30[6] | 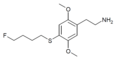
|
|||
| 2C-T-31[6] | 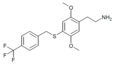
|
|||
| 2C-T-32[6] | 
|
|||
| 2C-T-33[6] | 
|
|||
| 2C-T-DFM | H | SCF2H | 
|
|
| CYB210010[8] | H | SCF3 | 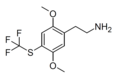
|
|
| 2C-T-DFP | H | SCH2CH2CF2H | 
|
|
| 2C-T-PARGY | H | SCH2C≡CH | 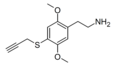
|
|
| 2C-DFM [9]: 770 | H | CHF2 | 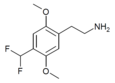
|
|
| 2C-TFM | H | CF3 | 
|
159277-08-4 |
| 2C-TFE | H | CH2CF3 | 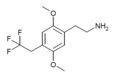
|
|
| 2C-PFE | H | CF2CF3 | 
|
|
| 2C-PFS | H | SF5 | 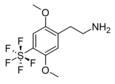
|
|
| 2C-YN | H | C≡CH | 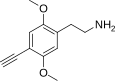
|
752982-24-4 |
| 2C-V | H | CH=CH2 | 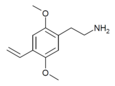
|
|
| 2C-AL[10] | H | CH2CH=CH2 | 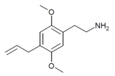
|
|
- ^ Alexander Shulgin, Tania Manning and Paul F Daley. The Shulgin Index. Volume 1. Psychedelic Phenethylamines and Related Compounds. Transform Press, 2011. ISBN 978-0-9630096-3-0
- ^ Daniel Trachsel, David Lehmann and Christoph Enzensperger. Phenethylamine Von der Struktur zur Funktion, pp 762-810. Nachtschatten Verlag AG, 2013. ISBN 978-3-03788-700-4
- ^ Shulgin, Alexander; Shulgin, Ann (September 1991). PiHKAL: A Chemical Love Story. Berkeley, California: Transform Press. ISBN 0-9630096-0-5. OCLC 25627628.
- ^ Takahashi M, Nagashima M, Suzuki J, Seto T, Yasuda I, Yoshida T. Creation and application of psychoactive designer drugs data library using liquid chromatography with photodiode array spectrophotometry detector and gas chromatography–mass spectrometry. Talanta, 15 Feb 2009, 77(4): 1245–1272. doi:10.1016/j.talanta.2008.07.062
- ^ Leth-Petersen S, Petersen IN, Jensen AA, Bundgaard C, Bæk M, Kehler J, Kristensen JL. 5-HT2A/5-HT2C receptor pharmacology and intrinsic clearance of N-benzylphenethylamines modified at the primary site of metabolism. ACS Chem. Neurosci., 16 Nov 2016, 7 (11), 1614–1619. doi:10.1021/acschemneuro.6b00265
- ^ a b c d e f g h i j k l m n o p q r s t "Shulgin's Sulfur Symphony – Part I". countyourculture. 15 January 2011. Archived from the original on 19 September 2019. Retrieved 22 October 2017.
- ^ Daniel Trachsel (2003). "Synthesis of novel (phenylalkyl)amines for the investigation of structure-activity relationships. Part 2. 4-Thio-substituted [2-(2,5-dimethoxyphenyl)ethyl]amines (=2,5-dimethoxybenzeneethanamines)". Helvetica Chimica Acta. 86 (7): 2610–2619. doi:10.1002/hlca.200390210.
- ^ Varty GB, Canal CE, Mueller TA, Hartsel JA, Tyagi R, Avery K, Morgan ME, Reichelt AC, Pathare P, Stang E, Palfreyman MG, Nivorozhkin A. Synthesis and Structure-Activity Relationships of 2,5-Dimethoxy-4-Substituted Phenethylamines and the Discovery of CYB210010: A Potent, Orally Bioavailable and Long-Acting Serotonin 5-HT2 Receptor Agonist. J Med Chem. 2024 Apr 25;67(8):6144-6188. doi:10.1021/acs.jmedchem.3c01961 PMID 38593423
- ^ Daniel Trachsel; David Lehmann & Christoph Enzensperger (2013). Phenethylamine: Von der Struktur zur Funktion. Nachtschatten Verlag AG. ISBN 978-3-03788-700-4.
- ^ Kruegel AC. Phenalkylamines and Methods of Treating Mood Disorders. Patent WO 2022/006186