 | |
 | |
| Clinical data | |
|---|---|
| Other names | MDEA, MDE, Eve |
| Routes of administration | Oral, insufflation, injection, rectal[1] |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Metabolism | Hepatic including CYP2D6 and CYP3A4 |
| Onset of action | 20–85 minutes |
| Elimination half-life | (R)-MDEA: 7.5 ± 2.4 hours (S)-MDEA: 4.2 ± 1.4 hours |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.231.031 |
| Chemical and physical data | |
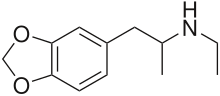
| Formula | C12H17NO2 |
| Molar mass | 207.273 g·mol−1 |
| 3D model (JSmol) | |
| |
3,4-Methylenedioxy-N-ethylamphetamine (MDEA; also called MDE and colloquially, Eve) is an empathogenic psychoactive drug. MDEA is a substituted amphetamine and a substituted methylenedioxyphenethylamine. MDEA acts as a serotonin, norepinephrine, and dopamine releasing agent and reuptake inhibitor.[1]
Possession of MDEA is illegal in most countries. Some limited exceptions exist for scientific and medical research.
- ^ a b Freudenmann RW, Spitzer M (2004). "The Neuropsychopharmacology and Toxicology of 3,4-methylenedioxy-N-ethyl-amphetamine (MDEA)". CNS Drug Reviews. 10 (2): 89–116. doi:10.1111/j.1527-3458.2004.tb00007.x. PMC 6741736. PMID 15179441.
- ^ Anvisa (2023-07-24). "RDC Nº 804 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 804 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 2023-07-25). Archived from the original on 2023-08-27. Retrieved 2023-08-27.