 | |
 | |
| Clinical data | |
|---|---|
| Other names | 5-Methoxy-N,N-dimethyltryptamine; 5-Methoxy-N,N-DMT; O-Methylbufotenin; Mebufotenin; Methylbufotenin; BPL-002; BPL-003; LSR-1019 |
| Routes of administration | Inhalation, insufflation, sublingual, intramuscular, intravenous, oral (with an MAOI)[1][2] |
| Drug class | Serotonergic psychedelic (hallucinogen) |
| ATC code |
|
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | Oral: inactive (without an MAOI) or weak[1][2] |
| Metabolism | Oxidative deamination (MAO), O-demethylation (CYP2D6)[2][1][4] |
| Metabolites | |
| Onset of action |
|
| Elimination half-life | Minutes[4] (12–19 min in mice)[2] |
| Duration of action |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.012.558 |
| Chemical and physical data | |
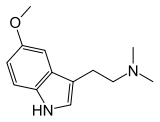
| Formula | C13H18N2O |
| Molar mass | 218.300 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
5-MeO-DMT (5-methoxy-N,N-dimethyltryptamine), also known as O-methylbufotenin or mebufotenin (INN), is a psychedelic of the tryptamine family.[1][4] It is found in a wide variety of plant species, and also is secreted by the glands of at least one toad species, the Colorado River toad. Like its close relatives dimethyltryptamine (DMT) and bufotenin (5-HO-DMT), it has been used as an entheogen in South America.[5] Slang terms include Five-methoxy, the power, bufo, and toad venom.[6]
The drug acts as a non-selective serotonin receptor agonist, including of the serotonin 5-HT1A and 5-HT2A receptors among others.[1][4] However, 5-MeO-DMT differs from most other serotonergic psychedelics in having 100- to 1,000-fold selectivity for the serotonin 5-HT1A receptor over the serotonin 5-HT2A receptor.[1][4] In relation to this, 5-MeO-DMT has been described as an "atypical" psychedelic and as producing subjective effects notably distinct from those of DMT and other psychedelics.[1][4] Like DMT, 5-MeO-DMT has a very rapid onset of action and short duration.[1][4]
- ^ a b c d e f g h i j k l m n Reckweg JT, Uthaug MV, Szabo A, Davis AK, Lancelotta R, Mason NL, Ramaekers JG (July 2022). "The clinical pharmacology and potential therapeutic applications of 5-methoxy-N,N-dimethyltryptamine (5-MeO-DMT)". J Neurochem. 162 (1): 128–146. doi:10.1111/jnc.15587. PMC 9314805. PMID 35149998.
- ^ a b c d e f g Shen HW, Jiang XL, Winter JC, Yu AM (October 2010). "Psychedelic 5-methoxy-N,N-dimethyltryptamine: metabolism, pharmacokinetics, drug interactions, and pharmacological actions". Curr Drug Metab. 11 (8): 659–666. doi:10.2174/138920010794233495. PMC 3028383. PMID 20942780.
- ^ Anvisa (2023-07-24). "RDC Nº 804 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 804 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 2023-07-25). Archived from the original on 2023-08-27. Retrieved 2023-08-27.
- ^ a b c d e f g h i Dourron HM, Nichols CD, Simonsson O, Bradley M, Carhart-Harris R, Hendricks PS (December 2023). "5-MeO-DMT: An atypical psychedelic with unique pharmacology, phenomenology & risk?". Psychopharmacology (Berl). doi:10.1007/s00213-023-06517-1. PMID 38072874.
- ^ Araújo AM, Carvalho F, Bastos M, Guedes de Pinho P, Carvalho M (August 2015). "The hallucinogenic world of tryptamines: an updated review". Archives of Toxicology. 89 (8): 1151–1173. doi:10.1007/s00204-015-1513-x. PMID 25877327. S2CID 4825078.
- ^ "Ultimate Guide to 5-MeO-DMT - Experience, Benefits, & Side Effects". 29 June 2020.