 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
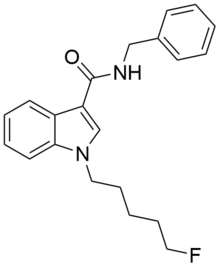
| Formula | C21H23FN2O |
| Molar mass | 338.426 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
5F-SDB-006 is a drug that acts as a potent agonist for the cannabinoid receptors, with an EC50 of 50 nM for human CB1 receptors, and 123 nM for human CB2 receptors.[1] It was discovered during research into the related compound APICA which had been sold illicitly as "2NE1".[2] 5F-SDB-006 is the terminally fluorinated analog of SDB-006, just as STS-135 is the terminally fluorinated analog of APICA. Given the known metabolic liberation (and presence as an impurity) of amantadine in the related compound APINACA, it is suspected that metabolic hydrolysis of the amide group of 5F-SDB-006 may release benzylamine.
- ^ Banister SD, Stuart J, Kevin RC, Edington A, Longworth M, Wilkinson SM, et al. (August 2015). "Effects of bioisosteric fluorine in synthetic cannabinoid designer drugs JWH-018, AM-2201, UR-144, XLR-11, PB-22, 5F-PB-22, APICA, and STS-135". ACS Chemical Neuroscience. 6 (8): 1445–58. doi:10.1021/acschemneuro.5b00107. PMID 25921407.
- ^ Banister SD, Wilkinson SM, Longworth M, Stuart J, Apetz N, English K, et al. (July 2013). "The synthesis and pharmacological evaluation of adamantane-derived indoles: cannabimimetic drugs of abuse". ACS Chemical Neuroscience. 4 (7): 1081–92. doi:10.1021/cn400035r. PMC 3715837. PMID 23551277.