| |
| Names | |
|---|---|
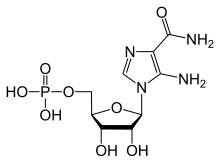
| IUPAC name
(1R)-1-(5-Amino-4-carbamoyl-1H-imidazol-1-yl)-1,4-anhydro-D-ribitol 5-(dihydrogen phosphate)
| |
| Systematic IUPAC name
[(2R,3S,4R,5R)-5-(5-Amino-4-carbamoyl-1H-imidazol-1-yl)-3,4-dihydroxyoxolan-2-yl]methyl dihydrogen phosphate | |
| Other names
AICAR, Aminoimidazole carboxamide ribonucleotide, AICA ribonucleotide, ZMP, 5-Amino-1-β-D-ribofuranosyl-imidazole-4-carboxamide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.019.285 |
| KEGG | |
| MeSH | AICA+ribonucleotide |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C9H15N4O8P | |
| Molar mass | 338.213 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
5-Aminoimidazole-4-carboxamide ribonucleotide (AICAR) is an intermediate in the generation of inosine monophosphate. AICAR is an analog of adenosine monophosphate (AMP) that is capable of stimulating AMP-dependent protein kinase (AMPK) activity. The drug has also been shown as a potential treatment for diabetes by increasing the metabolic activity of tissues by changing the physical composition of muscle.[1]
- ^ Zarembo, Alan (1 August 2008). "'Exercise pill' could take the work out of workouts". Los Angeles Times. Retrieved 21 January 2012.