 | |
| Identifiers | |
|---|---|
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C25H36O2 |
| Molar mass | 368.561 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
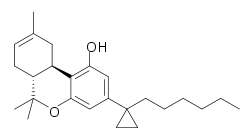
AMG-41 (part of the AM cannabinoid series) is an analgesic drug which is a cannabinoid agonist. It is a derivative of Δ8-THC substituted with a cyclopropyl group on the C1'-position of the C3-alkyl side chain. AMG-41 is a potent agonist at both CB1 and CB2, with a Ki of 0.44 nM at CB1 vs 0.86 nM at CB2.[1][2][3]
- ^ Papahatjis DP, Nikas SP, Andreou T, Makriyannis A (December 2002). "Novel 1',1'-chain substituted Delta(8)-tetrahydrocannabinols". Bioorganic & Medicinal Chemistry Letters. 12 (24): 3583–6. doi:10.1016/s0960-894x(02)00785-0. PMID 12443781.
- ^ Papahatjis DP, Nikas SP, Kourouli T, Chari R, Xu W, Pertwee RG, Makriyannis A (July 2003). "Pharmacophoric requirements for the cannabinoid side chain. Probing the cannabinoid receptor subsite at C1'". Journal of Medicinal Chemistry. 46 (15): 3221–9. doi:10.1021/jm020558c. PMID 12852753.
- ^ Papahatjis DP, Nahmias VR, Nikas SP, Andreou T, Alapafuja SO, Tsotinis A, et al. (August 2007). "C1'-cycloalkyl side chain pharmacophore in tetrahydrocannabinols". Journal of Medicinal Chemistry. 50 (17): 4048–60. doi:10.1021/jm070121a. PMID 17672444.