 | |
| Clinical data | |
|---|---|
| Pronunciation | /əˌbɛməˈsaɪklɪb/ ə-BEM-ə-SY-klib |
| Trade names | Verzenio, Verzenios, Ramiven, others |
| Other names | LY2835219 |
| AHFS/Drugs.com | Monograph |
| License data |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 45% |
| Protein binding | 96.3% |
| Elimination half-life | 18.3 hrs |
| Excretion | 81% via feces, 3% via urine |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.233.787 |
| Chemical and physical data | |
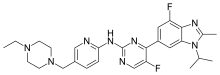
| Formula | C27H32F2N8 |
| Molar mass | 506.606 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Abemaciclib, sold under the brand name Verzenio among others, is a medication for the treatment of advanced or metastatic breast cancers. It was developed by Eli Lilly and it acts as a CDK inhibitor selective for CDK4 and CDK6.[4]
It was designated as a breakthrough therapy for breast cancer by the US Food and Drug Administration (FDA) in October 2015.[5]
In September 2017, it was approved for use in the United States by the FDA for the treatment of certain breast cancers.[6]
- ^ "Verzenio (Eli Lilly Australia Pty Ltd)". Therapeutic Goods Administration (TGA). 28 September 2022. Archived from the original on 18 March 2023. Retrieved 9 April 2023.
- ^ "Summary Basis of Decision (SBD) for Verzenio". Health Canada. 23 October 2014. Archived from the original on 31 May 2022. Retrieved 29 May 2022.
- ^ "Verzenios EPAR". European Medicines Agency. 27 September 2018. Retrieved 17 June 2024.
- ^ Lu J (August 2015). "Palbociclib: a first-in-class CDK4/CDK6 inhibitor for the treatment of hormone-receptor positive advanced breast cancer". Journal of Hematology & Oncology. 8 (1): 98. doi:10.1186/s13045-015-0194-5. PMC 4534142. PMID 26264704.
- ^ Digiulio S (8 October 2015). "FDA's Breakthrough Therapy Designation to Abemaciclib for Breast Cancer". Oncology Times. LWW Journals. Archived from the original on 4 June 2021. Retrieved 30 March 2016.
- ^ "FDA approves new treatment for certain advanced or metastatic breast cancers" (Press release). Food and Drug Administration. 28 September 2017. Archived from the original on 23 April 2019. Retrieved 29 September 2017.