 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Sectral, Prent, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a687003 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth, IV |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 40% (range 35 to 50%) |
| Metabolism | Hepatic |
| Elimination half-life | 3-4 hours (parent drug) 8-13 hours (active metabolite) |
| Excretion | Renal: 30% Biliary: 60% |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.048.654 |
| Chemical and physical data | |
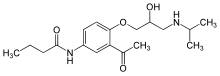
| Formula | C18H28N2O4 |
| Molar mass | 336.432 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 121 °C (250 °F) |
| |
| |
| (verify) | |
Acebutolol,[1] sold under the brand names Sectral among others, is a beta blocker for the treatment of hypertension and arrhythmias. Acebutolol is a cardioselective beta-1 blocker and has intrinsic sympathetic activity. It is commonly used in the treatment of angina.
It was patented in 1967 and approved for medical use in 1973.[2]
- ^ Elks J, Ganellin CR (1990). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 2–. ISBN 978-1-4757-2085-3.
- ^ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 461. ISBN 9783527607495.