 | |
| Clinical data | |
|---|---|
| Trade names | Hepsera |
| AHFS/Drugs.com | Monograph |
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 59% |
| Protein binding | <4% |
| Elimination half-life | 7.5 hours |
| Excretion | Urine |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| NIAID ChemDB | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.106.235 |
| Chemical and physical data | |
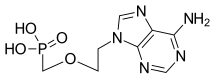
| Formula | C8H12N5O4P |
| Molar mass | 273.189 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Adefovir is a prescription medicine used to treat (chronic) infections with hepatitis B virus. A prodrug form of adefovir was previously called bis-POM PMEA, with trade names Preveon and Hepsera. It is an orally administered nucleotide analog reverse-transcriptase inhibitor (ntRTI). It can be formulated as the pivoxil prodrug adefovir dipivoxil.