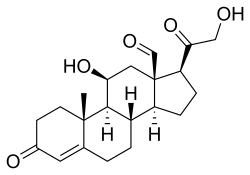
 Skeletal formula of the fictitious aldehyde form[1]
| |
 | |
| Names | |
|---|---|
| IUPAC name
11β,21-Dihydroxy-3,20-dioxopregn-4-en-18-al
| |
| Systematic IUPAC name
(1S,3aS,3bS,9aR,9bS,10S,11aR)-10-Hydroxy-1-(hydroxyacetyl)-9a-methyl-7-oxo-1,2,3,3a,3b,4,5,7,8,9,9a,9b,10,11-tetradecahydro-11aH-cyclopenta[a]phenanthrene-11a-carbaldehyde | |
| Other names
Aldocorten; Aldocortin; Electrocortin; Reichstein X; 18-Aldocorticosterone; 18-Oxocorticosterone
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.000.128 |
| KEGG | |
| MeSH | Aldosterone |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C21H28O5 | |
| Molar mass | 360.450 g·mol−1 |
| Pharmacology | |
| H02AA01 (WHO) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Aldosterone is the main mineralocorticoid steroid hormone produced by the zona glomerulosa of the adrenal cortex in the adrenal gland.[4][5] It is essential for sodium conservation in the kidney, salivary glands, sweat glands, and colon.[6] It plays a central role in the homeostatic regulation of blood pressure, plasma sodium (Na+), and potassium (K+) levels. It does so primarily by acting on the mineralocorticoid receptors in the distal tubules and collecting ducts of the nephron.[6] It influences the reabsorption of sodium and excretion of potassium (from and into the tubular fluids, respectively) of the kidney, thereby indirectly influencing water retention or loss, blood pressure, and blood volume.[7] When dysregulated, aldosterone is pathogenic and contributes to the development and progression of cardiovascular and kidney disease.[8] Aldosterone has exactly the opposite function of the atrial natriuretic hormone secreted by the heart.[7]
Aldosterone is part of the renin–angiotensin–aldosterone system. It has a plasma half-life of less than 20 minutes.[9] Drugs that interfere with the secretion or action of aldosterone are in use as antihypertensives, like lisinopril, which lowers blood pressure by blocking the angiotensin-converting enzyme (ACE), leading to lower aldosterone secretion. The net effect of these drugs is to reduce sodium and water retention but increase the retention of potassium. In other words, these drugs stimulate the excretion of sodium and water in urine, while they block the excretion of potassium.
Another example is spironolactone, a potassium-sparing diuretic of the steroidal spirolactone group, which interferes with the aldosterone receptor (among others) leading to lower blood pressure by the mechanism described above.
Aldosterone was first isolated by Sylvia Tait (Simpson) and Jim Tait in 1953; in collaboration with Tadeusz Reichstein.[10][11][12]
- ^ Singh N, Taibon J, Pongratz S, Geletneky C (2021). "Absolute content determination by quantitative NMR (qNMR) spectroscopy: a curious case of aldosterone". RSC Adv. 11 (38): 23627–23630. Bibcode:2021RSCAd..1123627S. doi:10.1039/D1RA03472C. PMC 9036601. PMID 35479823.
- ^ "CSD Entry: ALDAHA10". Cambridge Structural Database: Access Structures. Cambridge Crystallographic Data Centre. 1972. Retrieved 2022-09-03.
- ^ Duax WL, Hauptman H (1972). "Crystal structure and molecular conformation of aldosterone". J. Am. Chem. Soc. 94 (15): 5467–5471. doi:10.1021/ja00770a050. PMID 5040851.
- ^ Jaisser F, Farman N (January 2016). "Emerging Roles of the Mineralocorticoid Receptor in Pathology". Pharmacological Reviews. 68 (1): 49–75. doi:10.1124/pr.115.011106. PMID 26668301.
- ^ Marieb EN, Hoehn K (2013). "Chapter 16". Human anatomy & physiology (9th ed.). Boston: Pearson. pp. 629, Question 14. OCLC 777127809.
- ^ a b Arai K, Chrousos GP (2000-01-01). "Aldosterone Deficiency and Resistance". In De Groot LJ, Chrousos G, Dungan K, Feingold KR, Grossman A, Hershman JM, Koch C, Korbonits M, McLachlan R (eds.). Endotext. South Dartmouth (MA): MDText.com, Inc. PMID 25905305.
- ^ a b Marieb Human Anatomy & Physiology 9th edition, chapter:16, page:629, question number:14
- ^ Gajjala PR, Sanati M, Jankowski J (2015-07-08). "Cellular and Molecular Mechanisms of Chronic Kidney Disease with Diabetes Mellitus and Cardiovascular Diseases as Its Comorbidities". Frontiers in Immunology. 6: 340. doi:10.3389/fimmu.2015.00340. ISSN 1664-3224. PMC 4495338. PMID 26217336.
- ^ "Pharmacokinetics of Corticosteroids". 2003. Retrieved 15 June 2016.
- ^ Connell JM, Davies E (2005-07-01). "The new biology of aldosterone". Journal of Endocrinology. 186 (1): 1–20. doi:10.1677/joe.1.06017. ISSN 0022-0795. PMID 16002531.
- ^ Tait SA, Tait JF, Coghlan JP (2004-03-31). "The discovery, isolation and identification of aldosterone: reflections on emerging regulation and function". Molecular and Cellular Endocrinology. 217 (1–2): 1–21. doi:10.1016/j.mce.2003.10.004. PMID 15134795. S2CID 5738857.
- ^ Williams JS, Williams GH (June 2003). "50th anniversary of aldosterone". J Clin Endocrinol Metab. 88 (6): 2364–72. doi:10.1210/jc.2003-030490. PMID 12788829.