An alkylation unit (alky) is one of the conversion processes used in petroleum refineries. It is used to convert isobutane and low-molecular-weight alkenes (primarily a mixture of propene and butene) into alkylate, a high octane gasoline component. The process occurs in the presence of an acid such as sulfuric acid (H2SO4) or hydrofluoric acid (HF) as catalyst.[1] Depending on the acid used, the unit is called a sulfuric acid alkylation unit (SAAU) or hydrofluoric acid alkylation unit (HFAU). In short, the alky produces a high-quality gasoline blending stock by combining two shorter hydrocarbon molecules into one longer chain gasoline-range molecule by mixing isobutane with a light olefin such as propylene or butylene from the refinery's fluid catalytic cracking unit (FCCU) in the presence of an acid catalyst.[2][3]
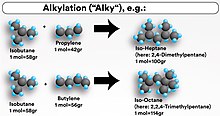
Since crude oil generally contains only 10-40% of hydrocarbon constituents in the gasoline range, refineries typically use an FCCU to convert high molecular weight hydrocarbons into smaller and more volatile compounds, which are then converted into liquid gasoline-size hydrocarbons. Byproducts of the FCC process also creates other low molecular-weight alkenes and iso-paraffin molecules which are not desirable. Alkylation transforms these byproducts into larger iso-paraffins molecules with a high octane number. While FCCUs are a very common unit in modern oil refineries, it is not common for a refinery to have an alkylation unit. Indeed, as of 2010 there are some countries in the world without any installed alkylation units.[4]
The product of the unit, the alkylate, is composed of a mixture of high-octane, branched-chain paraffinic hydrocarbons (mostly isoheptane and isooctane). Alkylate is a premium gasoline blending stock because it has exceptional antiknock properties and is clean burning. The octane number of the alkylate depends mainly upon the kind of alkenes used and upon operating conditions. For example, isooctane results from combining butylene with isobutane and has an octane rating of 100 by definition. There are other products in the alkylate effluent, however, so the octane rating will vary accordingly.[5]
- ^ "HF Alkylation Units". inspectioneering.com. Retrieved 2024-08-22.
- ^ "Alkylation Unit". McKinsey & Company. Retrieved October 16, 2019.
- ^ "Table 6: The results of optimal operation of alkylation unit problem". doi:10.7717/peerj-cs.1178/table-6.
{{cite web}}: Missing or empty|url=(help) - ^ "C₄ Isomerization, Alkylation & Olefins Hydrogenation | Axens". www.axens.net. Retrieved 2024-09-13.
- ^ Viswanathan, Balasubramanian (September 7, 2016). "2". Energy Sources: Fundamentals of Chemical Conversion Processes and Applications. Elsevier. doi:10.1016/C2011-0-05048-2. ISBN 978-0-444-56353-8. Retrieved October 17, 2019.