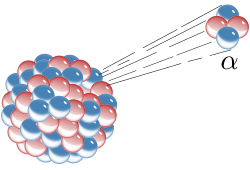
 | |
| Composition | 2 protons, 2 neutrons |
|---|---|
| Statistics | Bosonic |
| Symbol | α, α2+, He2+ |
| Mass | 6.6446573450(21)×10−27 kg[1] 4.001506179129(62) Da[2] 3.7273794118(11) GeV/c2[3] |
| Electric charge | +2 e |
| Spin | 0 ħ[4] |
Alpha particles, also called alpha rays or alpha radiation, consist of two protons and two neutrons bound together into a particle identical to a helium-4 nucleus.[5] They are generally produced in the process of alpha decay but may also be produced in other ways. Alpha particles are named after the first letter in the Greek alphabet, α. The symbol for the alpha particle is α or α2+. Because they are identical to helium nuclei, they are also sometimes written as He2+ or 4
2He2+ indicating a helium ion with a +2 charge (missing its two electrons). Once the ion gains electrons from its environment, the alpha particle becomes a normal (electrically neutral) helium atom 4
2He.
Alpha particles have a net spin of zero. When produced in standard alpha radioactive decay, alpha particles generally have a kinetic energy of about 5 MeV and a velocity in the vicinity of 4% of the speed of light. They are a highly ionizing form of particle radiation, with low penetration depth (stopped by a few centimetres of air, or by the skin).
However, so-called long-range alpha particles from ternary fission are three times as energetic and penetrate three times as far. The helium nuclei that form 10–12% of cosmic rays are also usually of much higher energy than those produced by nuclear decay processes, and thus may be highly penetrating and able to traverse the human body and also many metres of dense solid shielding, depending on their energy. To a lesser extent, this is also true of very high-energy helium nuclei produced by particle accelerators.
- ^ "2022 CODATA Value: alpha particle mass". The NIST Reference on Constants, Units, and Uncertainty. NIST. May 2024. Retrieved 18 May 2024.
- ^ "2022 CODATA Value: alpha particle mass in u". The NIST Reference on Constants, Units, and Uncertainty. NIST. May 2024. Retrieved 18 May 2024.
- ^ "2022 CODATA Value: alpha particle mass energy equivalent in MeV". The NIST Reference on Constants, Units, and Uncertainty. NIST. May 2024. Retrieved 18 May 2024.
- ^ Krane, Kenneth S. (1988). Introductory Nuclear Physics. John Wiley & Sons. pp. 246–269. ISBN 978-0-471-80553-3.
- ^ Bohan, Elise; Dinwiddie, Robert; Challoner, Jack; Stuart, Colin; Harvey, Derek; Wragg-Sykes, Rebecca; Chrisp, Peter; Hubbard, Ben; Parker, Phillip; et al. (Writers) (February 2016). Big History. Foreword by David Christian (1st American ed.). New York: DK. p. 58. ISBN 978-1-4654-5443-0. OCLC 940282526.