 | |
| Clinical data | |
|---|---|
| Trade names | Regumate, Matrix |
| Other names | Allyltrenbolone; Allyltrienolone; RU-2267; RH-2267; A-35957; A-41300; 17α-Allylestra-4,9,11-trien-17β-ol-3-one; 17α-Allyl-19-nor-δ9,11-testosterone |
| Routes of administration | By mouth[1] |
| Drug class | Progestogen; Progestin |
| ATCvet code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.011.549 |
| Chemical and physical data | |
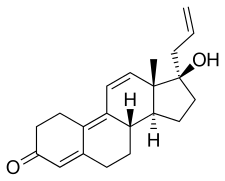
| Formula | C21H26O2 |
| Molar mass | 310.437 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Altrenogest, sold under the brand names Swinemate and Altren manufactured by Aurora Pharmaceutical and Regumate manufactured by Merck, is a progestin of the 19-nortestosterone group which is widely used in veterinary medicine to suppress or synchronize estrus in horses and pigs.[3][4][5][6][7] It is available for veterinary use in both Europe (as Regumate) and the United States (as Matrix).[8]
- ^ Asa CS (24 August 2005). "Types of contraception: The Choices". In Asa CS, Porton IJ (eds.). Wildlife Contraception: Issues, Methods, and Applications. JHU Press. pp. 35–. ISBN 978-0-8018-8304-0.
- ^ "Health product highlights 2021: Annexes of products approved in 2021". Health Canada. 3 August 2022. Retrieved 25 March 2024.
- ^ Elks J (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 35–. ISBN 978-1-4757-2085-3.
- ^ Index Nominum 2000: International Drug Directory. Taylor & Francis. January 2000. pp. 33–. ISBN 978-3-88763-075-1.
- ^ "Altrenogest". Drugs.com. Archived from the original on 2017-12-01. Retrieved 2017-11-25.
- ^ Reddy DS, Gadsby JE (13 May 2013). "Hormones Affecting Reproduction". In Riviere JE, Papich MG (eds.). Veterinary Pharmacology and Therapeutics. John Wiley & Sons. pp. 727–. ISBN 978-1-118-68590-7.
- ^ Zeelen FJ (1990). Medicinal chemistry of steroids. Elsevier Science Limited. pp. 108–109. ISBN 978-0-444-88727-6.
Other examples are allylestrenol (42), a pro-drug converted to the 3-keto analogue (43), which is used in the treatment of threatened abortion [78,79] and altrenogest (44), used in sows and mares to suppress ovulation and estrus behaviour [80]. [...] Progestins with a 17a-allyl side chain: (42) allylestrenol, (43), (44) altrenogest.
- ^ Rodriguez-Martinez H (1 April 2010). Control of Pig Reproduction VIII. Nottingham University Press. pp. 189–. ISBN 978-1-907284-53-3.