| |
| |
| Names | |
|---|---|
| IUPAC name
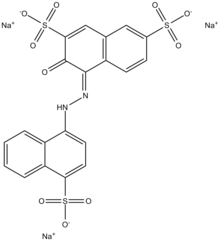
Trisodium (4E)-3-oxo-4-[(4-sulfonate-1-naphthyl)hydrazono]naphthalene-2,7-disulfonate
| |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.011.839 |
| EC Number |
|
| E number | E123 (colours) |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C20H11N2Na3O10S3 | |
| Molar mass | 604.47305 |
| Appearance | Dark red solid |
| Melting point | 120 °C (248 °F; 393 K) (decomposes) |
| Hazards | |
| GHS labelling: | |

| |
| Warning | |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P403+P233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Amaranth, FD&C Red No. 2, E123, C.I. Food Red 9, Acid Red 27, Azorubin S, or C.I. 16185 is a modified red azo dye used as a food dye and to color cosmetics. The name was taken from amaranth grain, a plant distinguished by its red color and edible protein-rich seeds.
Amaranth is an anionic dye. It can be applied to natural and synthetic fibers, leather, paper, and phenol-formaldehyde resins. As a food additive it has E number E123. Amaranth usually comes as a trisodium salt. It has the appearance of reddish-brown, dark red to purple water-soluble powder that decomposes at 120 °C without melting. Its water solution has an absorption maximum of about 520 nm. Like all azo dyes, Amaranth was, during the middle of the 20th century, made from coal tar; modern synthetics are more likely to be made from petroleum byproducts.[1][2]
Since 1976, amaranth dye has been banned in the United States by the Food and Drug Administration (FDA)[3] as a suspected carcinogen.[4][5] Its use is still legal in some countries, notably in the United Kingdom where it is most commonly used to give glacé cherries their distinctive color.
- ^ "Amaranth E123". LOOKCUT, INC. 2004–2010. Retrieved August 15, 2014.
- ^ "Aniline Wood Dye, Coal Tar Dyes". Craftsman Style. International Styles Network. 2005–2012. Retrieved August 15, 2014.
- ^ Compliance Program Guidance Manual: Domestic Food Safety (FY07-08) (PDF), Food and Drug Administration, November 9, 2008, p. 6,
Be aware that the following color additives are not on the list for use in food products in the United States. a. Amaranth (C.I. 16185, EEC No E123, formerly certifiable as FD&C Red No 2)
- ^ Color Additives Fact Sheet, U. S. Food and Drug Administration's Center for Food Safety and Applied Nutrition, July 30, 2001, archived from the original on November 17, 2001
- ^ Overview of Food Color Additives, Prepared for the USDA National Organic Program and the National Organic Standards Board, Agricultural Marketing Service (United States Department of Agriculture), October 14, 2005, pp. 1 and 7, archived from the original on March 9, 2010, retrieved August 15, 2014